
This is an Application Brief and does not contain a detailed Experimental section.
This application brief demonstrates method equivalency between the ACQUITY UPLC H-Class PLUS Binary System and ACQUITY UPLC System for the analysis of the USP method for budesonide.
In response to customer demand, the ACQUITY UPLC H-Class PLUS Binary System was developed to accommodate challenging gradients, providing a further increase in robust operation and throughput of methods utilizing the dynamic capabilities of the ACQUITY Binary Solvent Manager (BSM).
Liquid chromatography (LC) methods often need to balance many factors that include speed of analysis or throughput, sensitivity, selectivity, and robustness. Methods may further require the use of long, shallow gradients to ensure increased resolution of all analytes. This is often the case for peptide mapping analysis, as well as other types of profiling applications.
Analytical standard of budesonide was obtained from Sigma-Aldrich (Poole, UK). Benacort nasal spray (active ingredient – budesonide) was purchased over the counter of a local pharmacy. Separate preparations of 12.8 µg/mL budesonide standard and Benacort nasal spray were prepared for each system as per the USP method.3 Two separate lot numbers (i.e., 10833119915894/03403815715148) of the ACQUITY UPLC BEH C18, 1.7 µm, 2.1 x 50 mm columns (p/n: 186002350) were used for each analysis. The sample and standard were injected six times on each system to assess retention time, relative retention time (RRT), plate count, and resolution between the two LC platforms.
With these challenges in mind, the solution combines the ACQUITY UPLC Binary Solvent Manager (BSM) with the ACQUITY UPLC H-Class PLUS Sample Manager FTN, taking advantage of the low dwell volume and high-pressure mixing of the binary pump, and the larger bore internal tubing of the sample manager, which further increases robust operation.
As part of the method transferability assessment, consistent system performance needs to be established between the ACQUITY UPLC H-Class PLUS Binary System and existing chromatography platforms, both by isocratic and gradient utilization, the latter being covered in a separate application brief.1
In this application brief, we contrast the performance of the ACQUITY UPLC System which is equipped with a BSM, and the ACQUITY UPLC H-Class PLUS Binary System. The analysis of the isocratic UPLC method, converted directly from the USP method for budesonide,2 provides further confidence in successful method transfer between these two chromatography platforms.
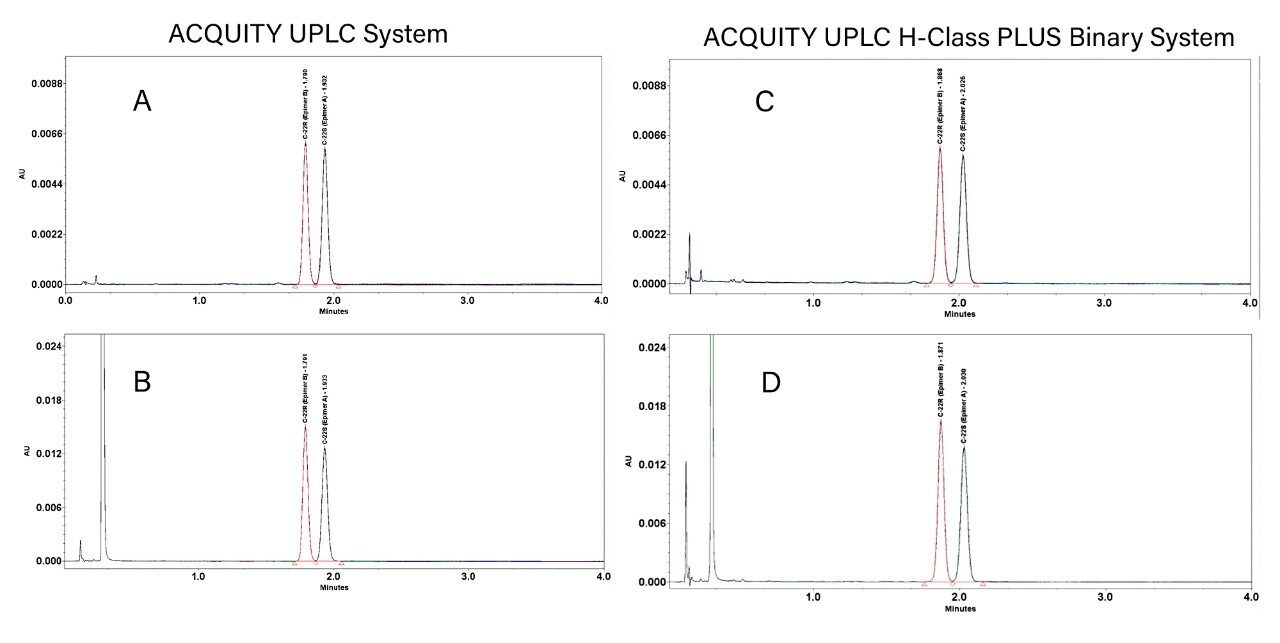
As the results show in tables 1 and 2, there is excellent agreement across all criteria tested with USP resolution between epimers identical to one decimal place.
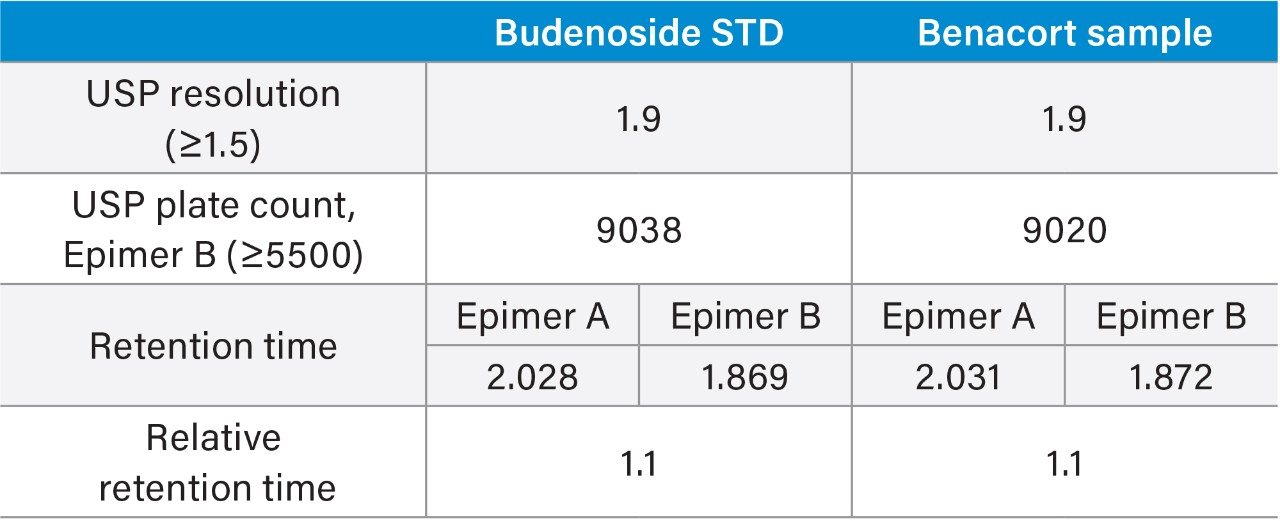
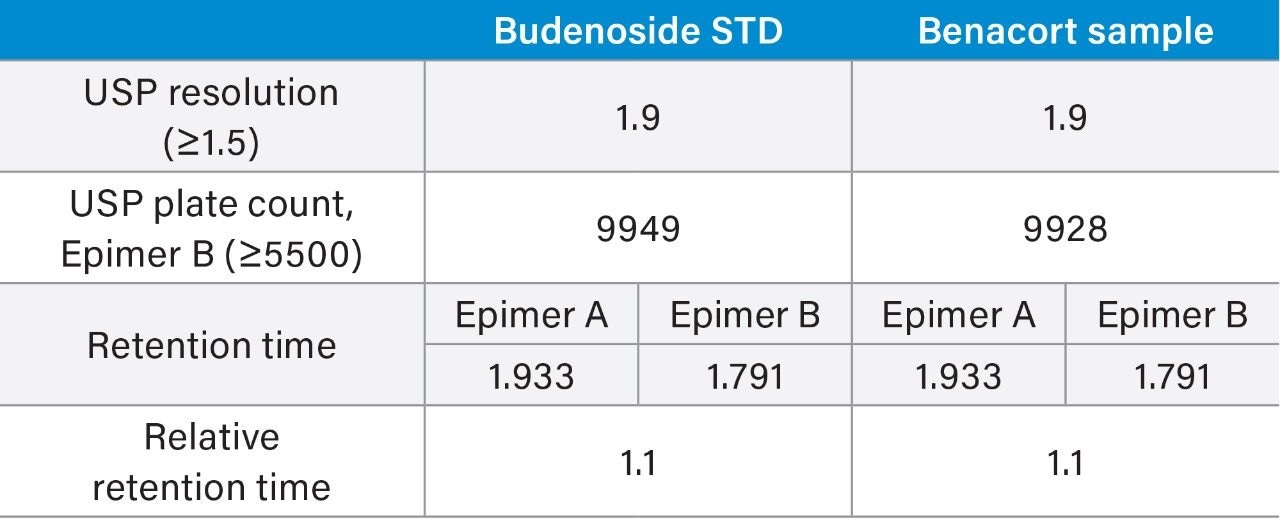
For the ACQUITY UPLC H-Class PLUS Binary System analysis, there is a slight increase in plate count for epimer B (approximately 10%) which can be attributed to the use of a new column. This would predictably exhibit greater column efficiency compared to an older/used column.
Retention time difference is less than 0.1 minutes between both platforms using separately-prepared mobile phase and two different column lot numbers. As this is an isocratic method, any variations in dwell volume do not affect retention time.
Relative retention times are consistent across both systems for sample and standard.
The isocratic UPLC method based on the original USP method for budenoside analysis was successfully transferred from the ACQUITY UPLC System to the ACQUITY UPLC H-Class PLUS Binary System.
All criteria assessments showed excellent agreement, easily satisfying any criteria laid down in a method transfer protocol, which is essential in a quality-controlled environment.
The ACQUITY UPLC H-Class PLUS Binary System has shown to be a demonstrably robust platform for transferring the described method, exhibiting consistent retention times, RRT’s, and resolution.
All system suitability results were satisfied according to the criteria laid down in the original USP method.
720006676, September 2019