This application note compares the use of both preparative RPLC and SFC techniques, discussing the scaling process from the analytical to preparative columns while illustrating the efficiency gains when using preparative SFC to isolate the impurities.
In the pharmaceutical industry, rapid preparative isolation is required for a number of reasons. These range from the use of chromatographic markers for the purpose of structure elucidation, for determination of relative response factors, and for reference standards. Current regulatory requirements on the control of impurities and degradation products in drug substances and drug product, dictate that for impurities at levels greater than 0.1%, unambiguous characterization is required.1 Modern, sophisticated, spectral hyphenated techniques, such as liquid chromatography mass spectrometry (LC-MS), liquid chromatography mass spectrometry-mass spectrometry (LC-MS/MS), and liquid chromatography nuclear magnetic resonance (LC-NMR), are already being extensively used by the industry, but in many instances, these techniques in isolation are not successful and an extra purification step is required to isolate the impurities for subsequent unambiguous characterization.2
Supercritical fluid chromatography (SFC) is a normal-phase chromatographic technique which found its place early in its development with chiral separations. In recent years, the improved quality of available hardware has led to a wider uptake of the technology for achiral separation applications, and has replaced reverse phase liquid chromatography (RPLC) separation techniques for the resolution of closely related species within the pharmaceutical and life science industries.3
The use of a super critical gas in chromatography, typically carbon dioxide due to the easily achievable conditions required to reach its critical point, (74 bar pressure and >32 °C temperature) offers several key benefits. Once above the critical point the liquid and gas phases are no longer distinct, meaning the super critical phase has solubilizing properties which are similar to a liquid with gas-like diffusivity. This translates chromatographically to lower back-pressures, allowing the use of higher flow rates, resulting in quicker analysis and more efficient chromatographic separations. SFC has also been praised as a ‘green’ separation technique eliminating the need for organic solvents such as heptane and hexane. Typical co-solvents used in SFC are alcohols (methanol, ethanol, or isopropanol) which are seen as a preferred solvent of choice because they are recognized by most as green chemistry initiatives.
For preparative and semi-preparative applications, the use of SFC allows the majority of the mobile phase to be a gas under normal temperatures and pressures, meaning that the resulting fractions are lower volume organic solutions. This reduces the time and cost associated with solvent evaporation. The use of SFC for achiral applications also avoids the use of pH modifiers, ion pair reagents, and buffers, all of which can be left as residues in final compounds or may interact with chemically sensitive analytes on concentration and dry down.
Traditionally dominated by reverse phase liquid chromatography (RPLC), there is an increasing trend toward using supercritical fluid chromatography (SFC) to replace RPLC for purifications from the semi-preparative scale up to the kilogram scale.4,5
In this application note we compare the use of both preparative RPLC and SFC techniques, discussing the scaling process from the analytical to preparative columns while illustrating the efficiency gains when using preparative SFC to isolate the impurities.
|
System: |
ACQUITY UPLC H-Class with ACQUITY UPLC PDA Detector |
|
Column: |
BEH C18 1.7 μm, 2.1 × 50 mm (P/N 186002350) |
|
Column temp.: |
50 °C |
|
Sample temp.: |
15 °C |
|
Injection volume: |
1 μL |
|
Flow rate: |
1 mL/min |
|
Mobile phase A: |
H2O |
|
Mobile phase B: |
MeCN +0.1% NH4OH |
|
Gradient: |
2–98% |
|
CSH C18, 1.7 μm, 2.1 × 50 mm, was also screened (P/N 186002140) |
|
|
Column: |
BEH C18 5 μm, 19 × 150 mm (P/N 186008166) |
|
Column temp.: |
50 °C |
|
Sample temp.: |
15 °C |
|
Injection volume: |
2.5 mL |
|
Flow rate: |
25 mL/min |
|
Mobile phase A: |
H2O |
|
Mobile phase B: |
Acetonitrile +0.1% NH4OH |
|
Gradient: |
50–60% |
|
Time: |
10 minutes |
Waters AutoPurification System: 2545 binary gradient module, 2767 sample manager, system fluidics organizer, 8 × 30 mL flow splitter, 2 × 515 pumps, 2998 photodiode array detector
|
Cone voltage: |
10 V |
|
Probe temp.: |
600 °C |
|
Ionization mode: |
ESI+ |
|
Sampling frequency: |
10 Hz |
|
Scan range: |
100–600 amu |
|
Wavelength: |
254 nm |
|
UPC2 method conditions |
|
|---|---|
|
System: |
ACQUITY UPC2 with ACQUITY UPC2 PDA Detector |
|
Column: |
TORUS 2-PIC 1.7 μm, 3 mm × 50 mm (P/N 186007600) |
|
Column temp.: |
45 °C |
|
Sample temp.: |
15 °C |
|
Injection volume: |
1 μL |
|
Flow Rate: |
2 mL/min |
|
Mobile Phase A: |
CO2 |
|
Mobile Phase B: |
Methanol |
|
Gradient: |
2–50% |
|
ABPR: |
1740 psi |
|
Other columns screened: |
Torus DIOL 1.7 μm, 3.0 × 50 mm (P/N 186007609) Torus DEA 1.7 μm, 3.0 × 50 mm (P/N 186007618) Torus 1-AA 1.7 μm, 3.0 × 50 mm (P/N 186007627) |
|
System: |
Investigator SFC System |
|
Column: |
Torus 2-PIC OBD Prep 5 μm, 10 mm × 150 mm (P/N 186008583) |
|
Column temp.: |
45 °C |
|
Sample temp.: |
15 °C |
|
Injection volume: |
0.5 mL |
|
Flow rate: |
15 mL/min |
|
Mobile phase A: |
CO2 |
|
Mobile phase B: |
Methanol |
|
Gradient: |
2–50% |
|
ABPR: |
1850 psi |
|
Time: |
4 minutes |
For RP prep 20 mg/mL in 90% water: 10% acetonitrile
For SFC prep 20 mg/mL in methanol
MassLynx Software v4.1 and ChromScope
For this study, the active pharmaceutical ingredient (API) fenofibrate was subjected to accelerated stressed conditions. When the resulting sample was analysed by LC-MS, two new impurities had been generated. RP-UPLC/MS analysis of the stressed material was performed on an ACQUITY UPLC H-Class System and ACQUITY QDa Mass Detector and the resulting chromatogram showed impurity 1 [M+H]+ 333, impurity 2 [M+H]+ 376, and the parent Figure 1A. The two new impurities elute before the main component and are just baseline resolved, limiting the sample loading which can be applied on the column.
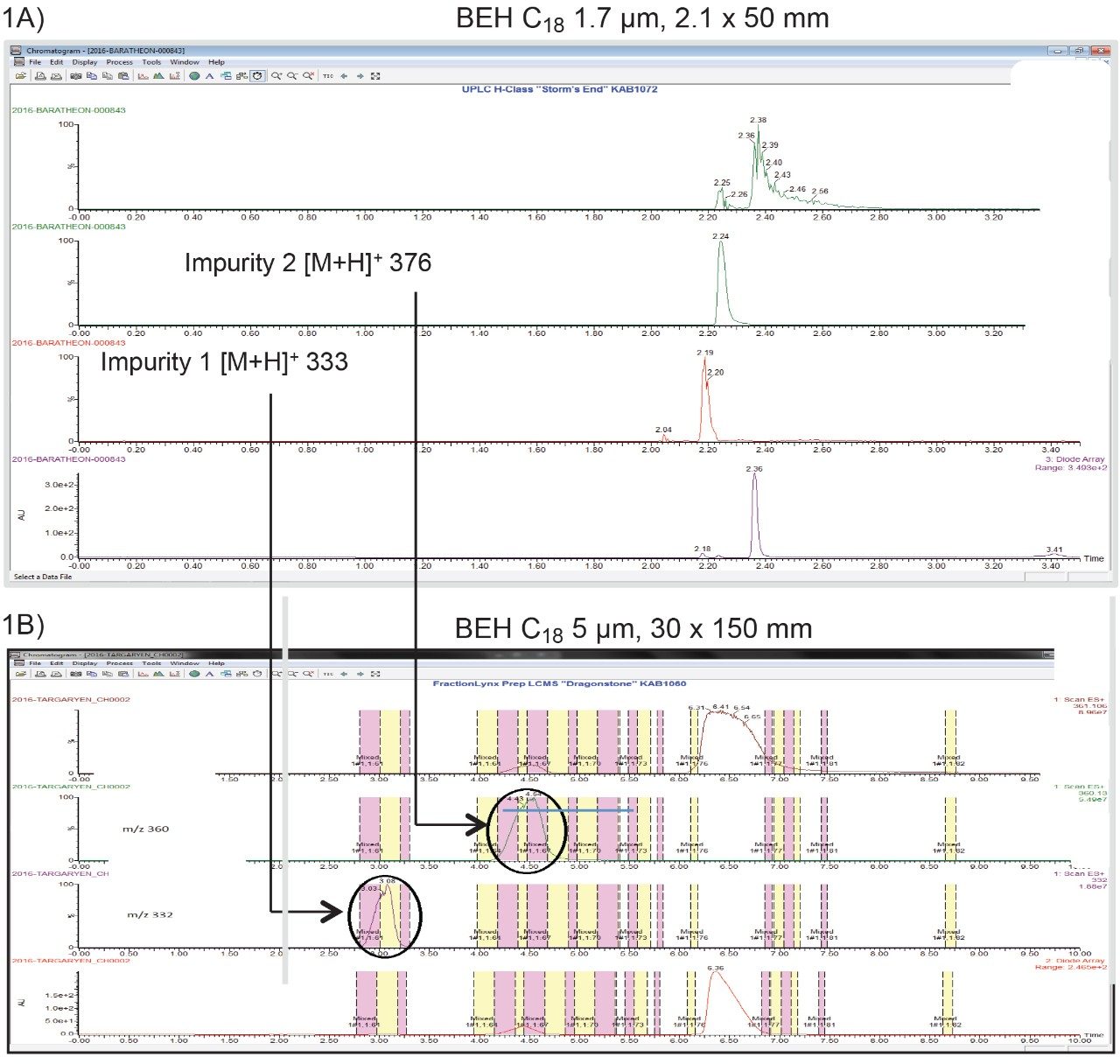
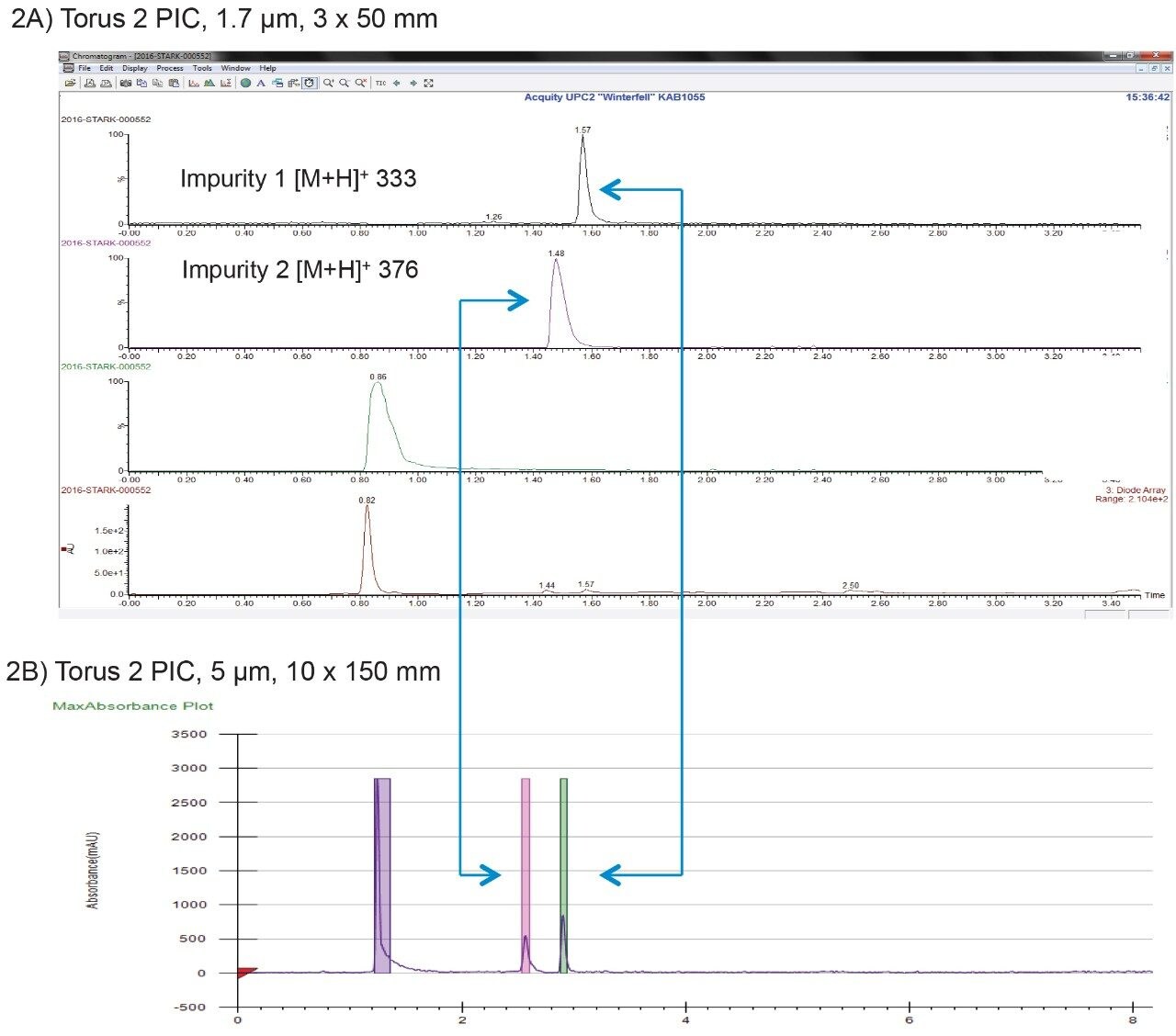
The comparative ACQUITY UPC2 System analysis, because of the different column selectivity, showed the main component eluting before the impurities Figure 2A. This allows for the collection of higher purity components with reduced loss of material. The scaling of analytical methods for preparative analysis is a well-documented process for reversed phase chromatography.6 Important factors to consider are:
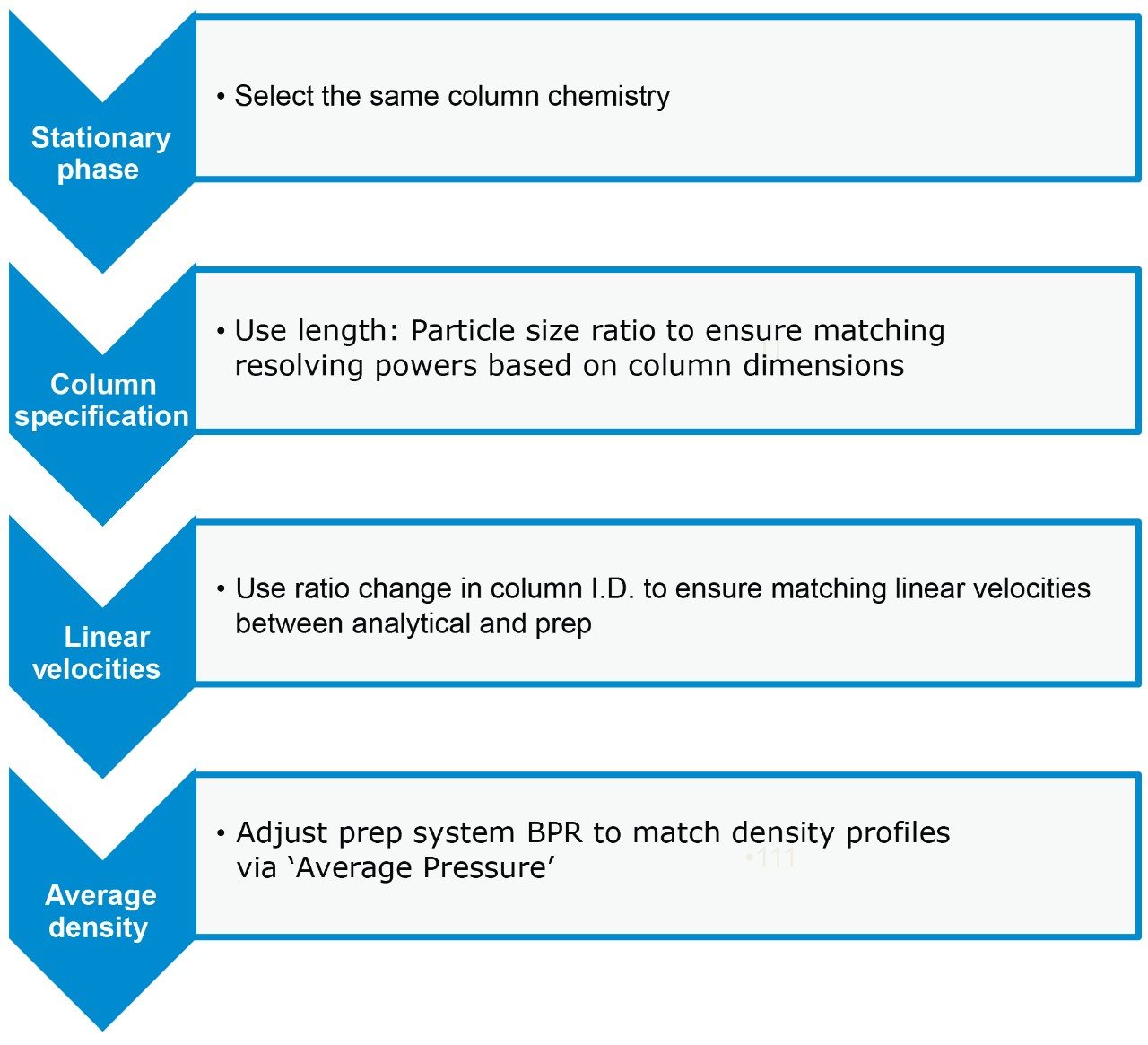
Many of the same principles for RPLC are applicable in SFC, but with the addition of one key parameter, density, which needs to be included to provide robust and reproducible scaling in SFC. The density profile of the chromatographic system varies naturally throughout a gradient run, as the proportions of co-solvent change, and because the supercritical fluid is, by its nature, a compressible fluid unlike reversed phase solvents. This affects the thermodynamic interactions in the mobile phase, and thus the retention times of analytes, and it is therefore crucial to match density profiles when transferring SFC methods.
This in turn poses a new challenge, as when moving from an analytical method to preparative method, a number of key properties change which will directly affect the density profile; these include changes in particle size, column length, and column I.D. Density profiles are, therefore, adjusted using the backpressure regulator setting to obtain as near to possible matching average pressures between preparative and analytical conditions. In practical terms, this usually means raising the preparative run back-pressure setting, as the larger column with large particle sizes acts to effectively increase the density within the system.6
In this application Waters Torus SFC columns on the analytical scale were initially screened with methanol and isopropanol for ‘best hit’ separations prior to optimization.
From this screening process, it was evident that the Torus 2-PIC column chromatogram was found to provide the selectivity and resolution required in Figure 2A. The method was transferred to the SFC Investigator System using a Torus 2-PIC 5 μm, 10 mm × 150 mm Column with methanol co-solvent and the separation is shown in Figure 2B. The scaled up method maintains the ratio between the column length and particle size, which is approximately 30,000 for both columns. The linear velocities between the analytical (3 mm I.D. and length 50 mm) and preparative column (10 mm I.D. and length 150 mm) were also preserved. In order to maintain the mobile phase density scaling from the analytical to preparative system, the average back-pressure regulator (ABPR) was adjusted from 1740 psi to 1850 psi. This analytical to preparative scaling process is summarized in the work flow shown in Figure 3.
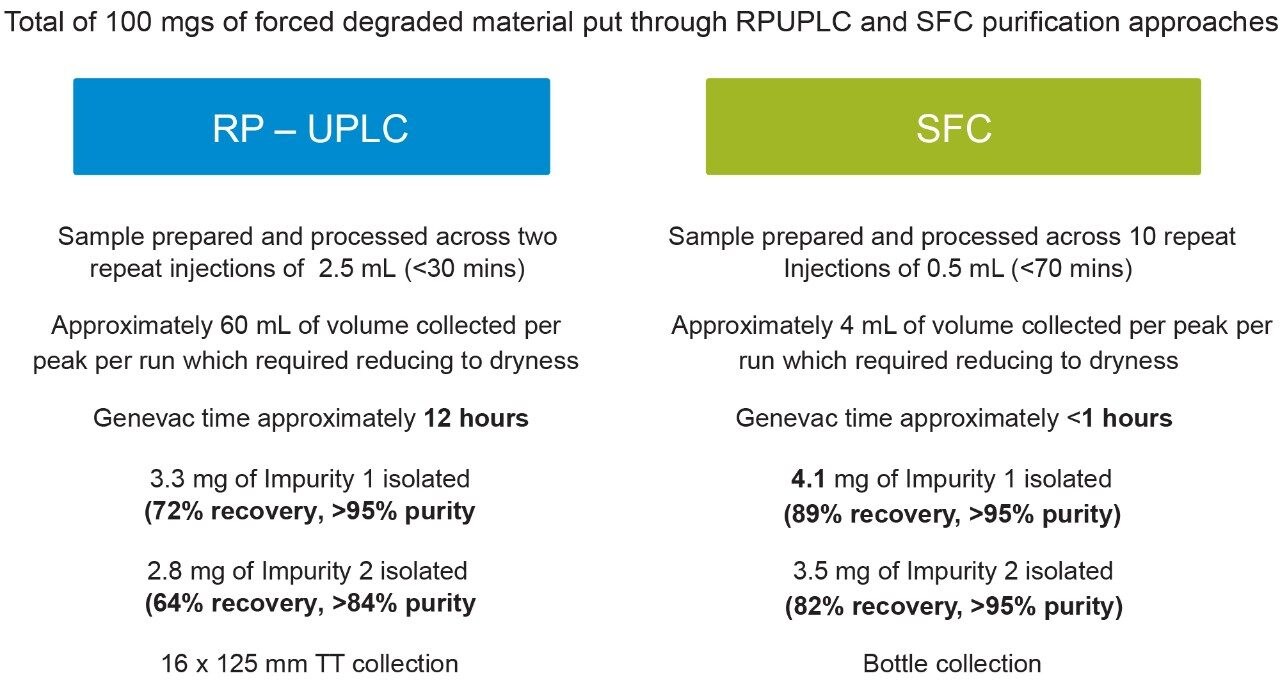
The column loading capacity depends on a number of variables which need to be assessed. These variables include the solubility and the resolution of the analytes involved in the separation, as well as the dimensions of the column. In this assessment, the 19 mm internal diameter (I.D.) preparative RP column has almost four times higher capacity than the 10 mm I.D. preparative SFC column. As a consequence, collection of the required amount of each impurity resulted in the RP experiments only requiring two repeat injections of 2.5 mL and the SFC prep requiring ten repeat 0.5 mL injections as seen in Figure 4. The effect of the lower flow rate of 11 mL/min, the peak width of 0.2 min, and a large portion of the eluent being CO2, the volume of solvent from each SFC run was 4 mL, totaling 40 mL which needed to be reduced to dryness for further work. Although the RP experiments only needed two repeat injections, a higher flow rate of 25 mL/min, the peak width of 0.5 minutes, and a completely liquid eluant resulted in 60 mL of solvent collected for each peak totaling 120 mL. The composition of the two extracts was different. The SFC extract contained only volatile methanol which was quickly reduced to dryness on a Genevac in less than one hour. In contrast, the RP extract was a mixture of nonvolatile aqueous buffers and acetonitrile which required twelve hours to reduce to dryness. The recoveries of the two impurities were higher from the SFC approach due to the sharper peak shape and the more favorable elution order.
Using the SFC approach enabled the entire purification to be completed in a time of two hours compared to 12.5 hours for the RP approach and used less than 63% of the solvent.
Full structural elucidation of the two impurities was successfully completed using a combination of HRMS and NMR studies (not reported herein).
720006327, July 2018