
This study aims to characterize sensitivity enhancements and reduction of matrix suppression for residue analysis. IonKey/MS offers some unique advantages for profiling complex matrices. Sensitivity enhancements enable sample dilution and hence matrix suppression reduction in residue screening assays, while maintaining data quality. It may be possible to reduce the need to closely matrix match QC samples to the test samples by introducing large sample dilution factors to negate the matrix load and thus benefit from efficiency savings within routine surveillance monitoring.
Pesticides have been widely used throughout the world since the middle of the 20th century. The Pesticide Manual Online lists information on more than 1,600 pesticides, with 10,400 product names, 3,100 discontinued products, and information for superseded materials believed to be no longer manufactured, or marketed for crop protection use. This information does set the scene for the challenge of pesticide residue analysis. The number of pesticides listed in the Pesticide Manual far exceeds the 357 pesticides approved for use within the EU.1 In addition, the regulations are constantly changing. For example, the impact of pesticide residues on bee populations has prompted legislation modification. The use of seed products containing the active plant protection substances clothianidin, thiamethoxam, and imidacloprid have been prohibited, within the constraints of approved conditions of use.2
Food commodities are sourced from a global network supply chain, therefore a residue screening strategy should be considered from a global perspective. Alder et al. indicated that there are more than 500 strictly regulated compounds that are routinely used and analyzed using GC-MS and LC-MS.3 With increasing global trade there is a need for qualitative multi-analyte screening strategies that are capable of efficiently detecting residue violations to protect consumer safety. Countries have different regulations concerning authorization of pesticides and Maximum Residue Limits (MRLs) established. The SANCO/12571/2013 guidance document describes the method validation and analytical quality control requirements to support the validity of data used for checking compliance with MRLs, enforcement actions, or assessment of consumer exposure to pesticides in the EU.4
Pesticide residue analysis in food has become a difficult task considering the increasing number of compounds and complex food commodities that need to be monitored at low concentrations with generic extraction procedures. The direct consequences are that complex extracts may include the presence of potentially interfering matrix components for which multiple injections may have to be performed, while achieving a dwell time and duty cycle balance.
Screening methods are a practical alternative, where the focus is primarily aimed towards qualitative detection in which neither requirements for recovery nor linearity are defined. Full scan High Resolution MS (HRMS) offers high specificity with, theoretically, no limitation in the number of compounds detected. Although time-of-flight mass spectrometry (Tof-MS) has provided the benefits of higher sensitivity and resolution, it is still a challenge to rapidly and efficiently identify targeted compounds in the presence of a large number of co-extracted matrix components. The benefits of full spectra acquisition and specificity of accurate mass measurement are well characterized and have been used in combination with retention time tolerances, isotope fits, fragment ions/ratios, and response thresholds to reduce false positive/negative identifications in non targeted screening assays. Also the ability to perform retrospective data review can be advantageous. From the mass spectral data generated, the challenge reverts to minimizing false detections through careful optimization of software screening parameters, while ensuring that when dealing with the impact of such complex matrices, false negative identifications do not result.
Over the last decade LC-MS has become the predominant approach for the analysis of small organic molecules, such as pesticide residues in food commodities. Multi-residue analysis utilizes generic sample extraction and chromatographic methodology; hence the analysis of very complex mixtures remains a challenge that the residue analyst has to deal with on a day-to-day basis. Recent advances in MS and separations technology, such as enhanced MS ion transmission and the increased peak capacity of UltraPerformance Liquid Chromatography (UPLC) have the potential to facilitate complex mixture analysis. StepWave, a unique off-axis ion source technology, provides additional sensitivity and increases the dynamic range required for routine pesticide screening. Such enhancements in Tof technology provide improved precision and accuracy in the data generated, but also necessitate the creation and utilization of more intelligent data processing software packages. Nonetheless, the ability to rapidly and efficiently identify targeted compounds present in a sample with a large number of co-extracted matrix components remains a challenge.
Advances in Waters’ mass spectrometry technology have vastly improved sensitivity for full spectral analysis, enabling Waters to provide the only unique fully validated pesticide screening solution.5 The drive to improve and develop new technology solutions continues in order to meet the ever changing requirements of residue analysis and more stringent regulations. Further sensitivity enhancements would help improve mass spectral data quality, which is especially important in order to avoid compromised precursor ion or fragment ion information and ensure high mass accuracy (≤2 mDa) below the legislated levels.
This study aims to characterize sensitivity enhancements and reduction of matrix suppression for residue analysis. The ionKey/MS System was comprised of the nanoACQUITY UPLC System,* the Xevo G2-S QTof Mass Spectrometer, the ionKey Source, and the iKey Separation Device, all controlled with MassLynx Software. Presented in Figure 1, the ionKey/MS System incorporates UPLC separation into the mass spectrometer source, delivering exceptional performance and a simplified user experience.

Figure 1. The ionKey Source, which incorporates the ACQUITY UPLC Peptide BEH C18 Column and ionization emitter.
*Replaced by the ACQUITY UPLC M-Class System.
|
LC system: |
nanoACQUITY UPLC* |
|
Mobile phase A: |
100% Water, 0.1% Formic acid |
|
Mobile phase B: |
100% Acetonitrile, 0.1% Formic acid |
|
Flow rate: UPLC: |
450 μL/min |
|
iKey: |
1 μL/min |
|
Injection volume: |
UPLC: 5 μL iKey: 2 μL |
|
UPLC column: |
ACQUITY UPLC Peptide BEH C18, 130Å 1.7 μm, 2.1 mm x 100 mm (p/n 186003555) |
|
Column temp.: |
30 °C |
|
Separation device: |
iKey Peptide BEH C18, 300Å, 1.7 μm, 150 μm x 100 mm (p/n 186006970) |
|
iKey temp.: |
45 °C |
*Replaced by the ACQUITY UPLC M-Class System.
|
Time (min) |
Flow rate (μL/min) UPLC |
Flow rate (μL/min) iKey |
%A |
%B |
|---|---|---|---|---|
|
0.00 |
450 |
1 |
98.0 |
2.0 |
|
0.25 |
450 |
1 |
98.0 |
2.0 |
|
12.25 |
450 |
1 |
1.0 |
99.0 |
|
13.00 |
450 |
1 |
1.0 |
99.0 |
|
13.01 |
450 |
1 |
98.0 |
2.0 |
|
13.00 |
450 |
1 |
98.0 |
2.0 |
|
17.00 |
450 |
1 |
98.0 |
2.0 |
|
MS system: |
Xevo G2-S QTof |
|
Ionization mode: |
ESI+, conventional probe and ionKey Source |
|
Desolvation temp.: |
550 °C (UPLC) |
|
Mass range: |
0 to 1,200 Da |
|
Acquisition rate: |
10 spectra/s |
|
Capillary voltage: |
1 kV |
|
Cone voltage: |
20 V |
|
Collision energy ramp: |
10 to 45 eV |
|
Resolution: |
30,000 (FWHM) |
|
Lockmass: |
m/z 556.2766 (Leucine enkephalin) |
The assay was based on the analysis of solvent standards in addition to matrix samples: organic mandarin, ginger, leek, and pear extracts, plus matrix matched calibrants.
10 g of homogenized sample was extracted with 60 mL of 20 mM ammonium acetate in methanol using an Ultra-Turrax device. Then, the crude extract was filtered and diluted up to 100 mL with 5 mM ammonium acetate in water prior to injection.
Organic samples were homogenized and 10 g was extracted with 60 mL of 20 mM acetate ammonium in a methanol/water (95:5; v/v) solution. Then 5 mL and 3 mL of raw extract were transferred to six volumetric flasks. For the spiking of 0.01, 0.05, 0.1 mg/kg levels, 50, 250, 500 µL respectively of a mix solution were added containing the targeted pesticides at 0.1 µg/mL. For the higher levels, 0.2, 0.5, and 1.0 mg/kg were added (100, 250, and 500 µL respectively) of a mix solution containing the targeted pesticides at 1 µg/mL. Then the final volumes were adjusted to 5 mL with 5 mM ammonium acetate in water/methanol (90/10; v/v).
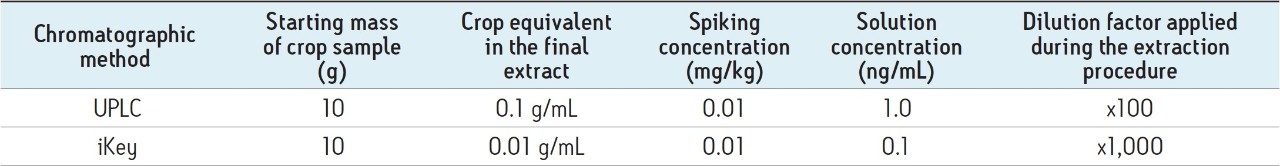
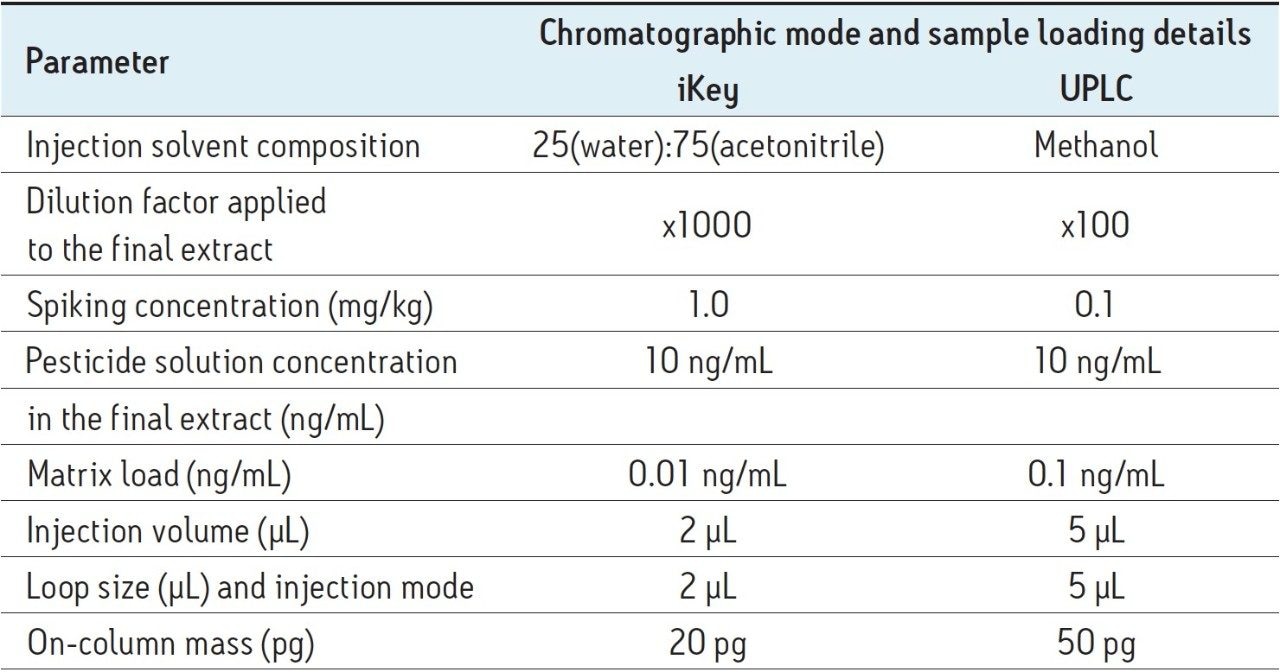
In order to take into consideration the different injection volumes and sample dilutions, on-column loadings were used in order to generate extrapolated comparative results. For the ionKey/MS System reduction matrix suppression studies, samples were diluted using 25% water:75% acetonitrile.
The iKey Separation Device, shown in Figure 2, incorporates a 1.7 µm, ACQUITY UPLC BEH C18 Column (p/n 186003555), stationary phase in a 150 μm diameter separation channel. The iKey Separation Device temperature was set to 45 °C, and the eluent from the separation channel flows directly to the integrated ESI emitter. All microfluidic, gas, and electrical connections are automatically engaged when the iKey Separation Device is inserted into the source enclosure and the handle is turned locking it into place.

Data were processed using MassLynx Software and the UNIFI Scientific Information System. Peak volume was determined using UNIFI’s 3D peak detection algorithm.
A direct comparison of UPLC and the ionKey/MS System for screening of pesticide residues in food was performed to explore the potential benefits of iKey microfluidic chromatography combined with time-of-flight mass spectrometry for residue analysis purposes. Analysis of mandarin, pear, leek, and ginger extracts, for pesticide residues was performed. The acquired MassLynx data were processed with the UNIFI Scientific Information System, which has been specifically designed for non-targeted accurate mass screening applications. An ionKey Source (Figure 1) with the integrated iKey (Figure 2), was interfaced to a Xevo G2-S QTof Mass Spectrometer, where the acquisition of precursor and fragment ions (MSE) was performed. Improvements in ionization transmission efficiency produced using the ionKey/MS System can be observed in Figure 3. The proximity of the iKey Separation Device emitter to the sampling cone orifice allows the finer, smaller droplets produced to enter the mass spectrometer. Using conventional electrospray, even with visual inspection, the droplet sizes in the plume were larger and also only a part of the plume was sampled.

In addition to the improved transmission efficiency using the iKey Separation Device, an increase in ionization efficiency was also observed. Figure 4 shows the linearity and dynamic range obtained for imazalil in a mandarin extract. A correlation coefficient of R2=0.994 was obtained for 0.1 ng/mL to 100 ng/mL (0.1 pg/μL-100 pg/μL) using the ionKey Source. Figure 5 shows the limit of detection (LOD), where both precursor and retention time aligned fragment ion information was obtained for imazalil, at 500 fg/μL level in vial.

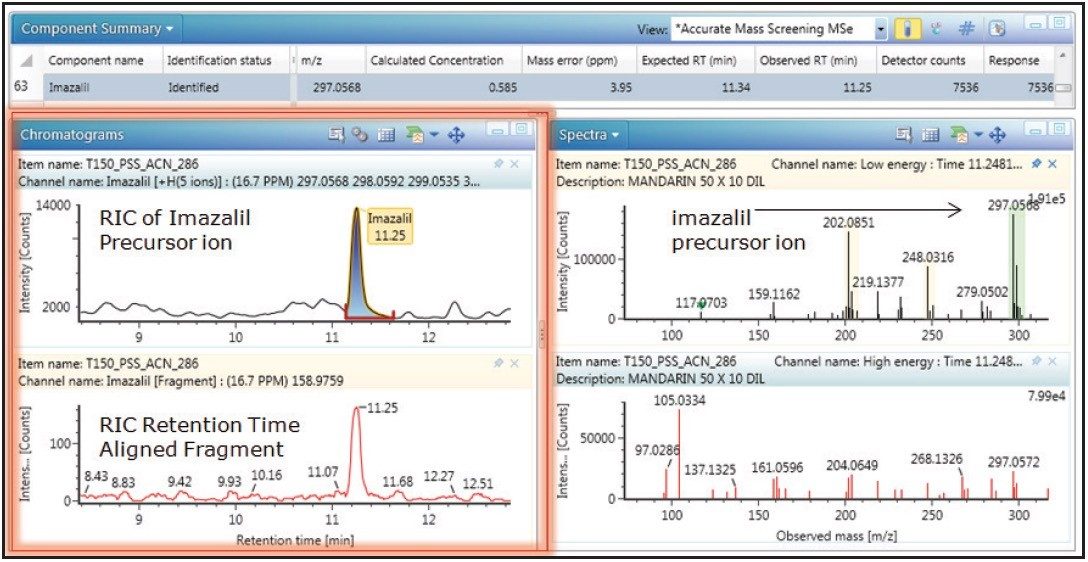
The characteristic imazalil isotope distribution is highlighted in green. A further example of fortified mandarin extract is presented for thiabendazole in Figures 6 and 7. A correlation coefficient of R2=0.999 was obtained over a concentration range of 0.1 ng/mL to 100 ng/mL (0.1 pg/μL to 100 pg/µL); also both precursor and retention time aligned fragment ion information is illustrated for thiabendazole.


It is significant to note that for imazalil and thiabendazole, LODs of 100 fg/μL in vial were obtained; precursor ion data only was achieved in this case. Both precursor ion and fragment ions were obtained at 500 fg/μL in vial. Mandarin, pear, leek, and ginger fortified extracts were analyzed, and acceptable linearity was observed for all four matrices, with R2’s of the order of 0.95 or above. Typical correlation coefficients obtained for pesticides in the matrix matched samples analyzed are presented in Table 3.

The results presented for thiabendazole/imazalil clearly illustrate the spectral quality and linearity that can be obtained using the ionKey/MS System. LODs of 100 fg/µL in vial have been determined using time-of-flight full spectral analysis. In order to determine how the increased sensitivity and exceptional system performance has been generated, comparison of data from the pesticide solvent standards and matrix matched samples was undertaken.
During the electrospray process, creation of droplets with an excess of positive charges occurs. Ionization efficiency can be impacted by a number of factors such as flow rate, interface design, solvent composition, buffer concentration, matrix composition, or analyte properties (polar/non-polar). In general, as the eluent flow to the electrospray emitter decreases, ionization efficiency increases because of an increase in the production of smaller charged droplets at lower flow rates.
In this study, optimum sensitivity was determined at 1 µL/min. Electrospray current in cone-jet mode (when a liquid meniscus held at the exit of a metallic capillary tube is charged to a high voltage, the free surface often takes the form of a cone whose apex emits a steady micro jet) increases approximately as the square root of the volumetric flow rate; therefore the number of available charges per analyte molecule increases as the flow rate decreases. This can be explained if droplet size is considered. For example, 1,000 droplets with a 1 μm diameter have the same volume as one droplet with a diameter of 10 μm. However the surface area of the 1,000 droplets is 10 times higher, than that of the 10 μm diameter droplet. Hence small droplets are able to carry a higher percentage of charge. Smaller initial droplets and increased amount of charge available per analyte molecule improve the ionization of analytes with lower surface activity, improving quantitation and reducing matrix suppression effects. More efficient solvent evaporation results from smaller droplet sizes and as a result, fewer coulombic fission events are required to create gas phase ions.6-16
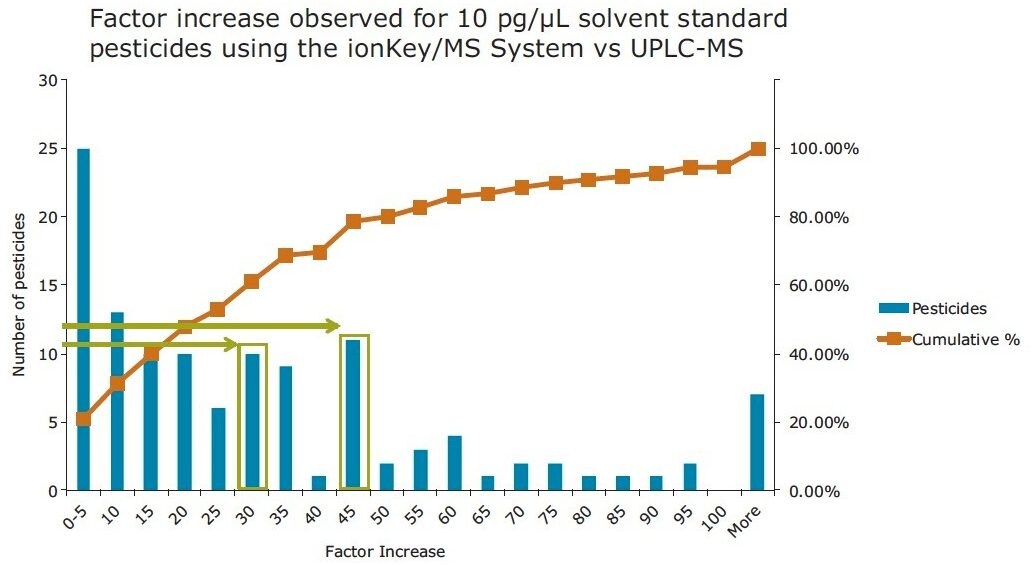
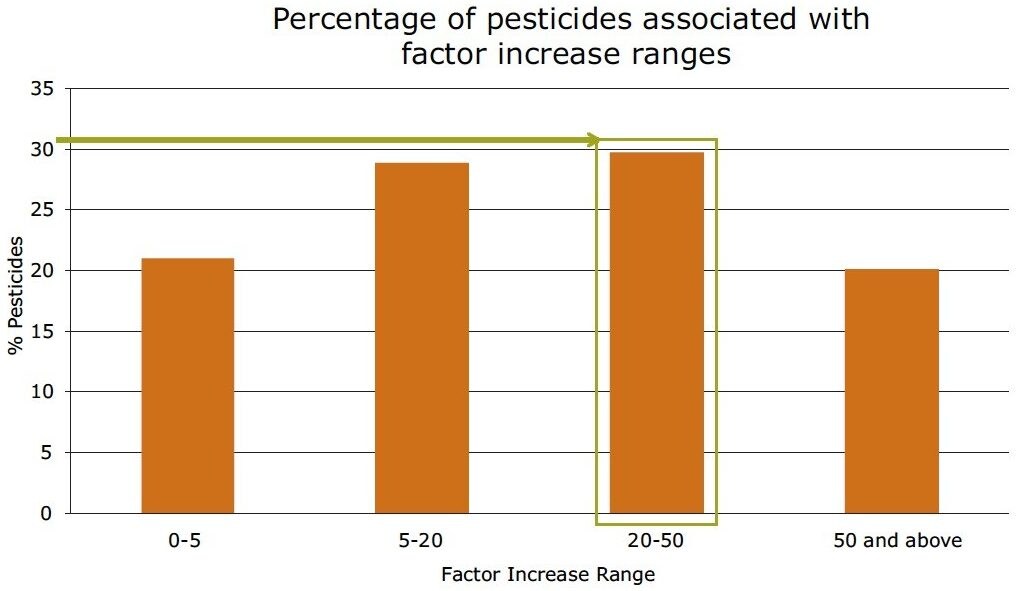
In Figure 8, the improvement in sensitivity achieved using the ionKey/MS System is shown. The responses for pesticides in solvent at 10 pg/µL in vial, were compared using the optimized UPLC-MS and the ionKey/MS System conditions. The response factor increase of the ionKey/MS System to standard UPLC-MS is shown on the X axis and the bars correspond to the number of pesticides that demonstrated the factor increase. The red curve shows the cumulative percentage of total pesticides in the analysis. It can be observed from the data that the factor increase produced by the ionKey/MS System for 80% (identified from the cumulative frequency curve) of the pesticides was up to x45, with the final 20% having a factor increase of > x45 in response. The factor increase in response (3D peak volume) does vary and is compound dependent. Aldicarb sulfone, diafenthiaurion, fenpyroximate, and isofenphos-methyl exhibited the lowest response factor increases, while linuron, chlorbromuron, and dichlorvos exhibited the highest response factor increases. Figure 9 shows a summary of the information in Figure 8. Overall, approximately 30% of the pesticides have a factor increase in response of 20 to 50 when using the ionKey/MS System compared UPLC-MS for solvent standards. The injection volumes for UPLC (5 µL) and iKey (2 µL) should also be noted for this comparison and the response obtained using the ionKey/MS System has been extrapolated to take into consideration the different injection volumes.
Typical screening parameters can include the precursor ion (with adducts), fragment ions, retention time, mass accuracy (precursor ion and fragments), and isotope ratios. The accuracy of LC-MS measurements can be influenced by matrix effects during atmospheric pressure ionization, which inevitably can impact the detection results. IUPAC defines matrix effects as “the combined effect of all components of the sample other than the analyte on the measured quantity”.17 Ionization suppression is believed to occur because of the number of excess charges and the limited space on the surface of the charged droplets produced in the ion source. Competition for surface position and charge can be dominated by matrix components over analytes. Solvent evaporation and Raleigh fission can also be inhibited because of increased surface tension and viscosity caused by the matrix.
Advantages and limitations of approaches to compensate for matrix effects in multi-class and multi-residue analyses were discussed by Lehotay et al.18 These include the separation of large matrix components for multiple analytes, matrix removal without impacting analyte recoveries, and the requirements of many blank extracts. The impact of dilution on final extracts is also discussed, along with improved quantification limits.
Stanke et. al., performed a systematic study of the “dilute and shoot” approach for reducing matrix suppression with the desire to determine a relationship between matrix concentration and suppression effect.19 This work also used generic extraction techniques, which are convenient, but contain high matrix concentrations. Stanke concluded that matrix can cause significant suppression of analytes. Using a 10-fold dilution, suppression resulting from generic extraction techniques, such as QuEChERS, can be reduced by 25% to 50%. In order to remove matrix effects, a 100-fold dilution step, or alternatively, a sample extraction method achieving 99% matrix removal would be required. Conventional ESI source designs may have a slight influence on suppression, but would not affect the principal relationship between matrix effect and matrix suppression.18 The Granby extraction method was utilized in this collaborative project, where unlike QuEChERS, no sample cleanup step is performed.20
In order for an analyte to appear in the mass spectrum it must successfully compete for a place on the charged surface of the droplets. The extent of matrix effects depends on the ability of the matrix to occupy the surface of a droplet. With transmission at low flow rates that the iKey Separation Device design enables, samples can now be diluted further to minimize matrix suppression, while still attaining the required LODs for contaminant analysis. To assess what this might mean for pesticide analysis, a comparison between UPLC-MS System and the ionKey/MS System was undertaken in spiked matrix samples. The analysis of the solvent standards presented here captures the ionization improvements that can be gained using the ionKey/MS System. The improvement in sensitivity allows for the dilution of the matrix within the sample preparation workflow. In order to capture the combined impact of both ionization improvement and the option for matrix dilution, the concentration of the analytes was kept constant while diluting the matrix component for the ionKey/MS System experiment. Therefore, a solution concentration of 10 pg/μL was used for both the UPLC-MS and the ionKey/MS System measurements. Table 2 summarizes the sample information used for the direct comparison of the iKey and UPLC chromatographic systems.
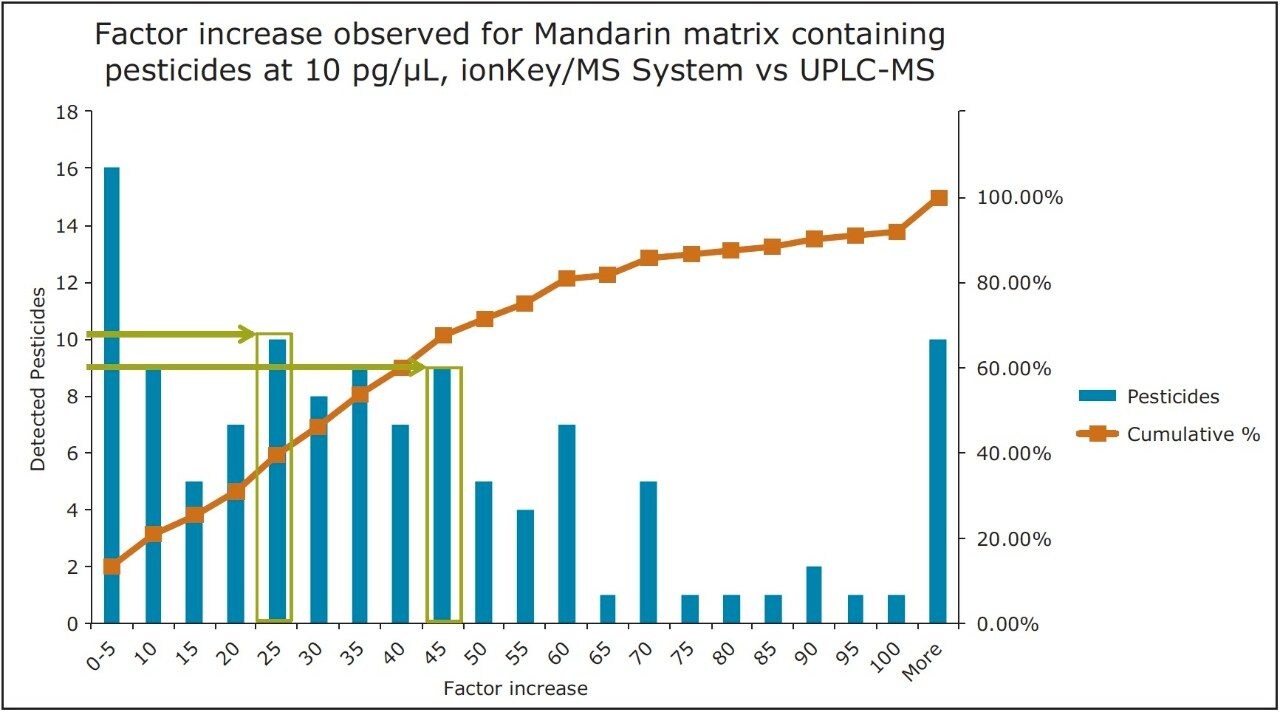
The pesticide residue response for fortified mandarin matrix is presented in Figure 10. For 80% of the pesticides (identified from the cumulative frequency curve), the factor increase produced using the iKey Separation Device was up to x60. The final 20% have a factor increase of > x60 in response. The increase in response does vary and is known to be dependent on the physicochemical properties of the compound. This is highlighted by the varied increases in response in Figure 10, where 10 pesticides in mandarin matrix have a factor increase of x25, and a factor increase of x45 was observed for another 9.
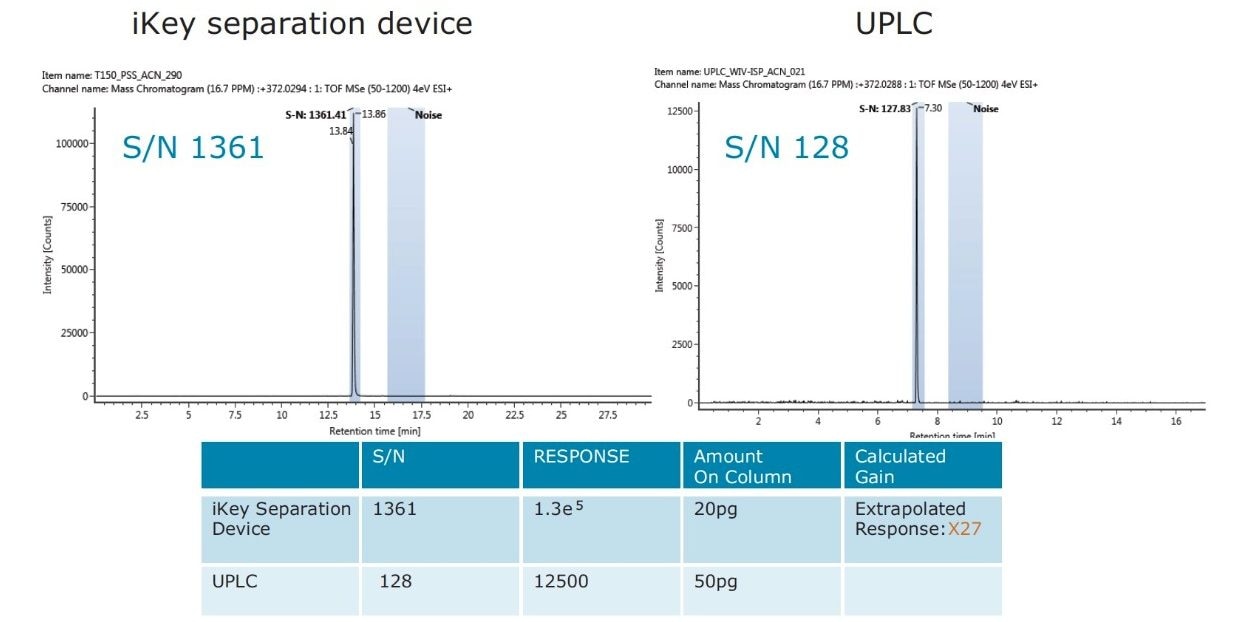
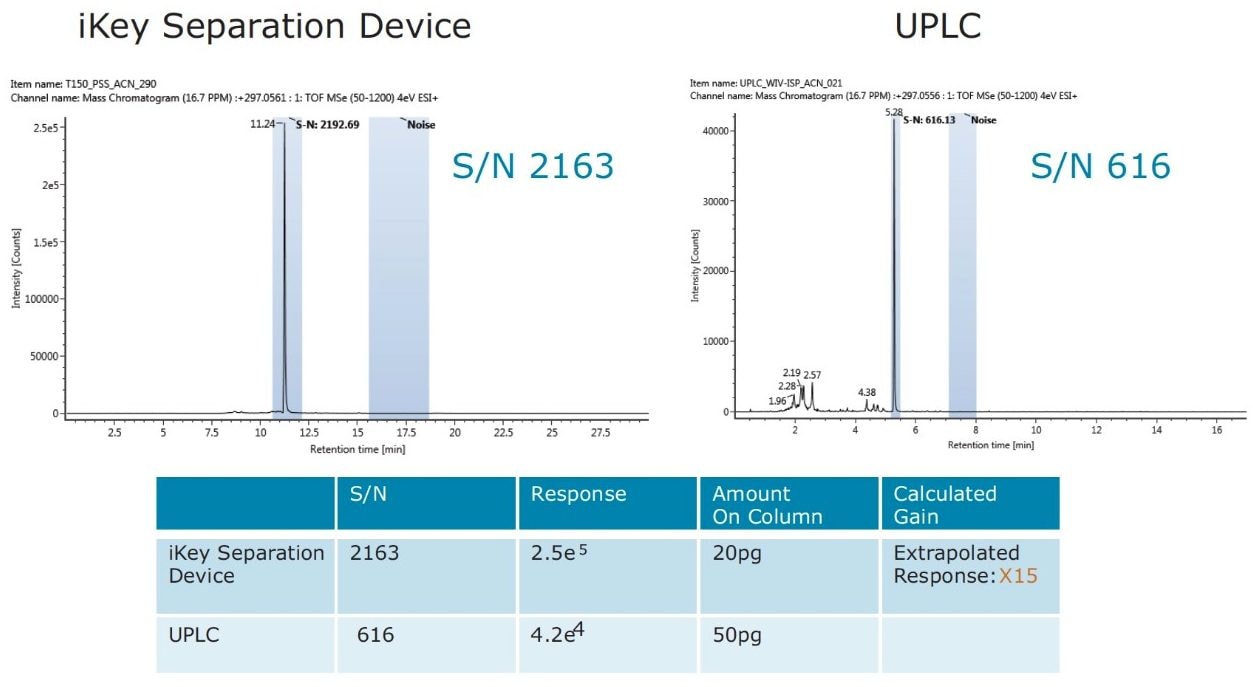
The impact of matrix suppression reduction is also apparent when comparing signal-to-noise (S/N). In Figure 11, a comparison of S/N measured for tetraconazole using UPLC-MS and the ionKey/MS System is illustrated. The S/N was determined for pesticide residues spiked into mandarin matrix at 10 pg/μL in vial (UPLC) and 100 pg/μL in vial x10 dilution (iKey). The concentration injected on column in each case was therefore 10 pg/μL. For UPLC, a S/N of 128 was obtained, compared to a S/N of 1361 using the iKey Separation Device. This is a 10x improvement in S/N. The injection volumes for UPLC (5 µL) and iKey Separation Device (2 µL) should also be noted for this comparison. Even with 2.5 times more injected on UPLC, the ionKey/MS System gave a 10x increase in S/N. Comparison of S/N for imazalil is presented in Figure 12, where for UPLC, a S/N 616 was obtained, compared to a S/N of 2163 for the ionKey/MS System, which equates to a x3.5 improvement in S/N. Variations in the S/N and response improvement reflect the extent to which matrix suppression has been reduced, as well as different improvements in ionization efficiency for each of the analytes. To compare the responses obtained for imazalil for example, using UPLC (5 μL injection) and iKey Separation Device (2 μL injection), the iKey Separation Device absolute response was multiplied by 2.5 and divided by the UPLC absolute response.
Data acquired from solvent standards and mandarin matrix samples were compared so that the effect of dilution on the matrix effects could be investigated for both chromatographic systems. The factor difference of pesticide solvent standards (the ionKey/MS System versus UPLC-MS) shows the improvement predominantly due to ionisation efficiency. The factor difference of mandarin matrix samples (the ionKey/MS System versus UPLC) shows improvement from both ionisation efficiency and the reduction of matrix effects (due to x10 dilution). By comparing the improvements observed in both experiments, we can gauge the improvement that comes from the difference due to matrix effect alone.
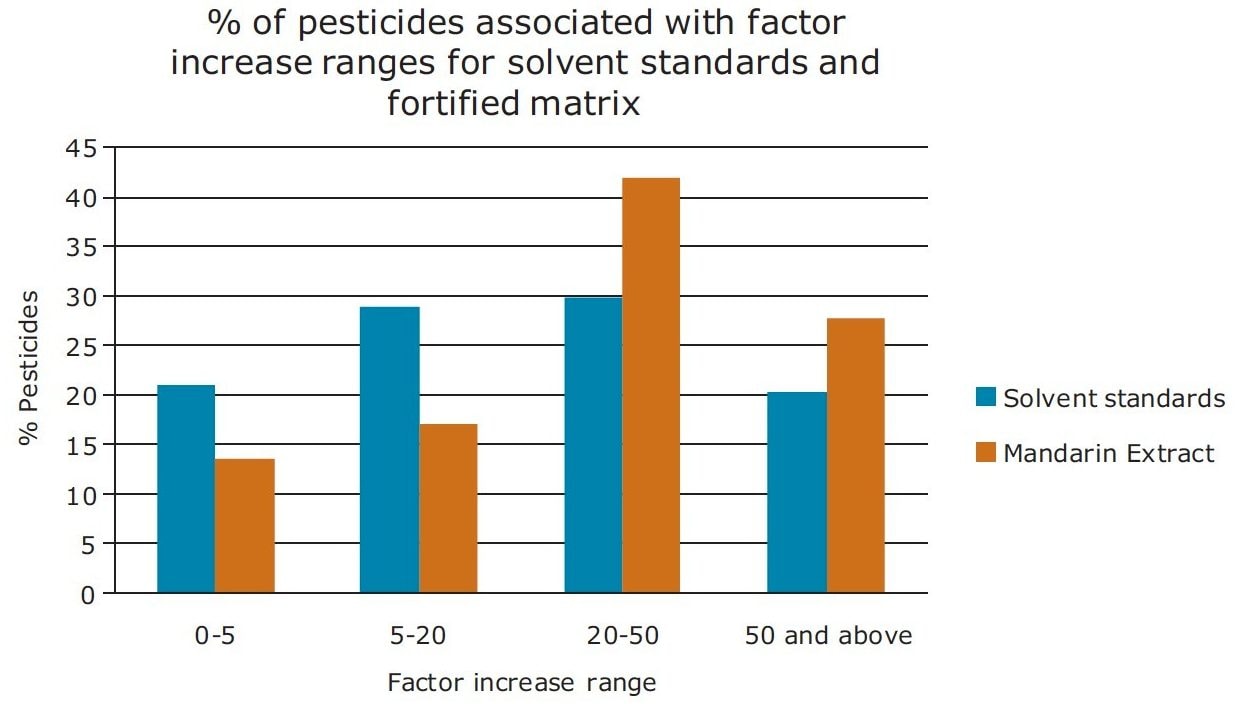
In Figure 13, a summary of the percentage of pesticides associated with factor increase using the ionKey/MS System compared to UPLC-MS for pesticide solvent standards and diluted mandarin extracts is presented. In the diluted mandarin matrix sample, 42% of the pesticides experienced a 20 to 50-fold increase in response. This compared to 30%for the solvent standards. These data indicate that matrix suppression was reduced for a large number of the pesticides, while an increase in sensitivity using the ionKey/MS System was observed.
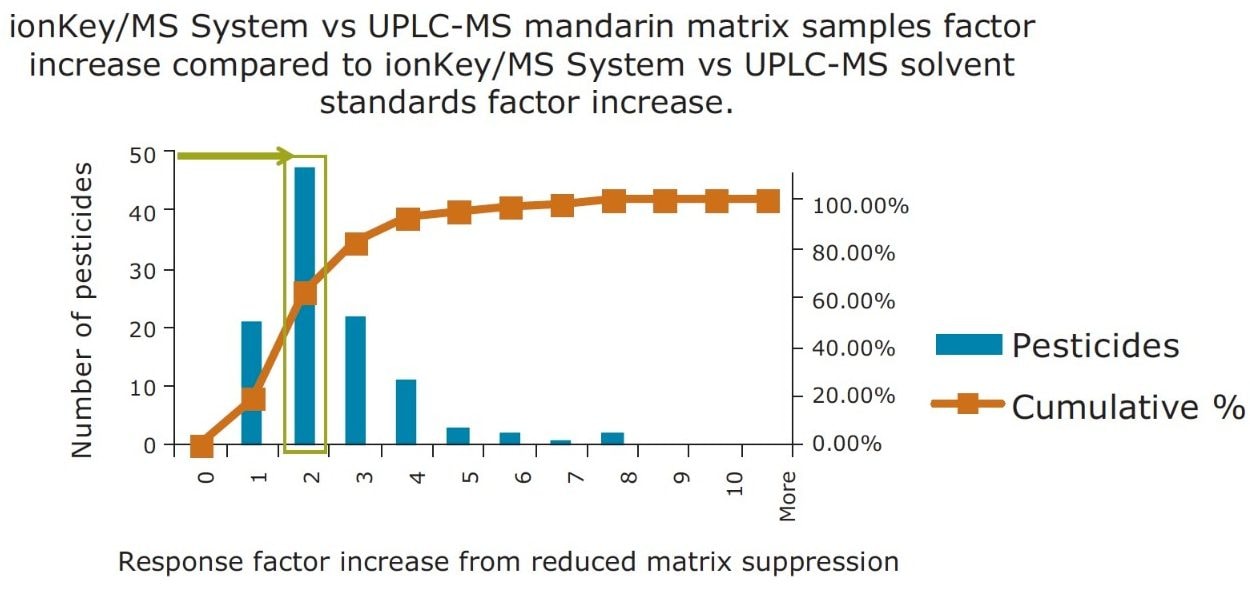
In Figure 14, a comparison of the response gains obtained for the solvent standards and those obtained in the diluted matrix is summarized in a statistics histogram chart, which estimates the probability of distribution of a continuous variable. For the 48 pesticides detected in the mandarin matrix, a factor of 2 increase in sensitivity was observed. An improvement is clearly observed for 80% of the pesticides, with an additional factor of x2 or better improvement in the ionKey/MS System response due to the reduction of matrix suppression.
It is clear that the ionKey/MS System can improve absolute sensitivity and help to reduce the challenges of low level residue detection in food matrices. The matrices analyzed are complex and representative of challenging food commodities. The ability to dilute sample matrix and still obtain excellent response and S/N of analytes was demonstrated using the ionKey/MS System. The robust design of the ionKey Source enables the analyst to explore microfluidic chromatography as a routine analytical tool. The ionKey/MS System offers an improvement in data quality at and below required legislative LODs.
720005195, January 2016