
This application note describes a method of extensive charge reduction of native protein complexes on unmodified SYNAPT G2 and SYNAPT G2-S Ion Mobility Mass Spectrometers.
The production of high-mass, multiply charged ions via electrospray ionization (ESI) permits mass spectrometric analysis of native protein complexes.1,2 However, given the relatively broad peaks typical in native MS, mass and charge state assignment can be challenging, particularly for heterogeneous samples.3 Furthermore, a correlation exists between protein charge state and conformation, and lower charge states are often believed to resemble the native (solution) state more closely.4 Therefore, methods of reducing the gas-phase charge state offer analytical benefits. Previous efforts have generally either relied on ejection of highly charged monomers,3 causing significant disruption of the native structure, or have required instrument modification.5
Here, we describe a method of extensive charge reduction of native protein complexes on unmodified SYNAPT G2 and SYNAPT G2-S Ion Mobility Mass Spectrometers. This results in increased peak separation and simplified spectral assignment, as illustrated in Figure 1, but also allows us to investigate electrostatic effects on protein folding state by using ion mobility. Concomitantly, some ETD fragments, originating from surface-exposed terminal regions, are also observed, providing sequence information.
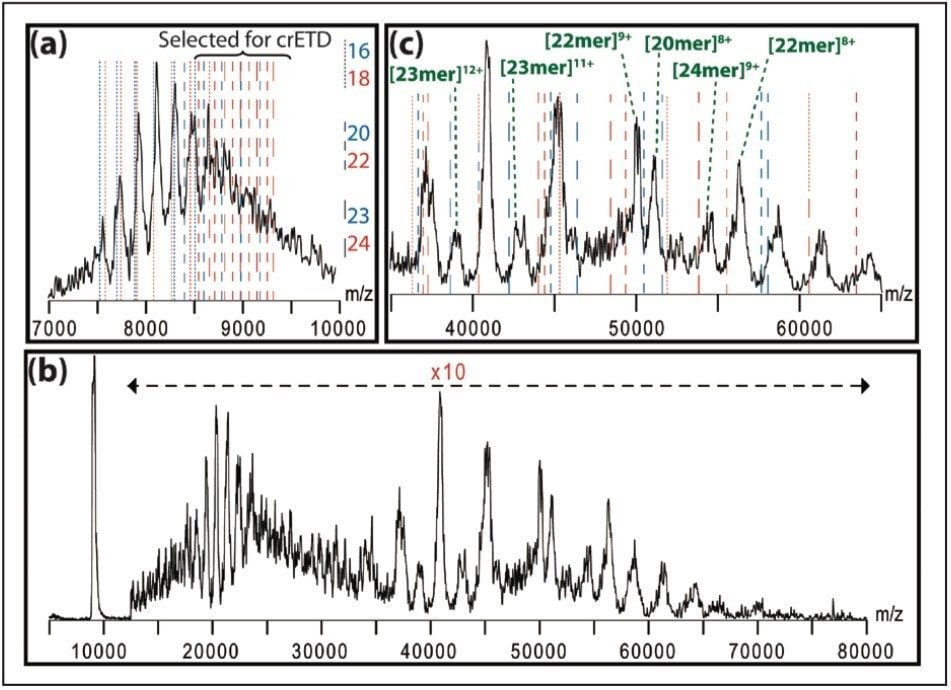
Proteins were dissolved at a concentration of 1 mg/mL in 100 mM aqueous ammonium acetate (pH = 6.8) and desalted twice using Bio-Rad Micro Bio-Spin 6 columns.
Samples were introduced via direct infusion. Both 1,4-dicyanobenzene and p-nitrotoluene were used as ETD reagents, yielding similar results.
|
MS systems: |
SYNAPT G2 and SYNAPT G2-S |
|
Capillary voltage: |
1.0 kV |
|
Trap pressure: |
8e-2 mbar |
|
Trap DC bias: |
11 V (45 V in Mobility mode) |
|
Transfer pressure: |
6e-3 mbar (2e-2 mbar in Mobility mode), transfer CE 5 V |
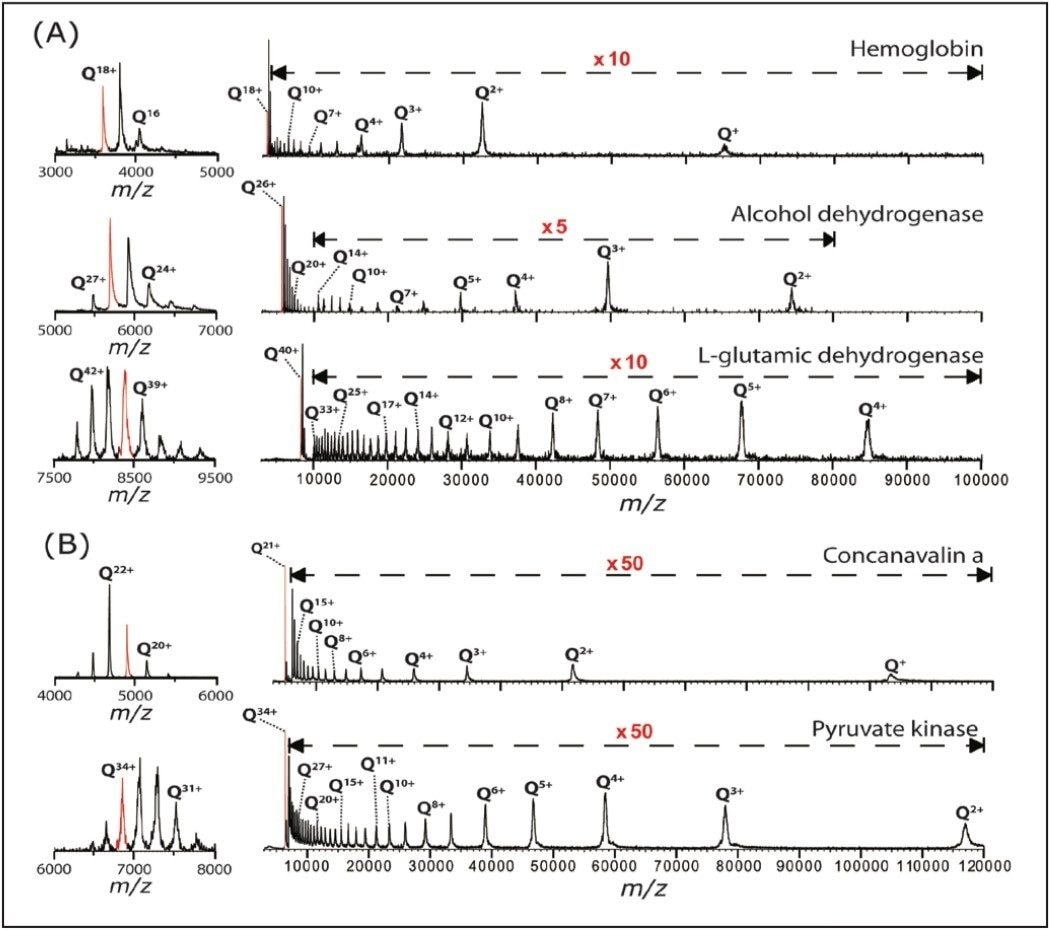
Besides the polydisperse αB-crystallin, shown in Figure 1, we also tested this method on five monodisperse complexes: hemoglobin (64 kDa), concanavalin a (103 kDa), alcohol dehydrogenase (ADH, 148 kDa), pyruvate kinase (233 kDa), and L-glutamic dehydrogenase (GDH, 336 kDa). As shown in Figure 2, this leads to the observation of extensive charge reduction in all cases.
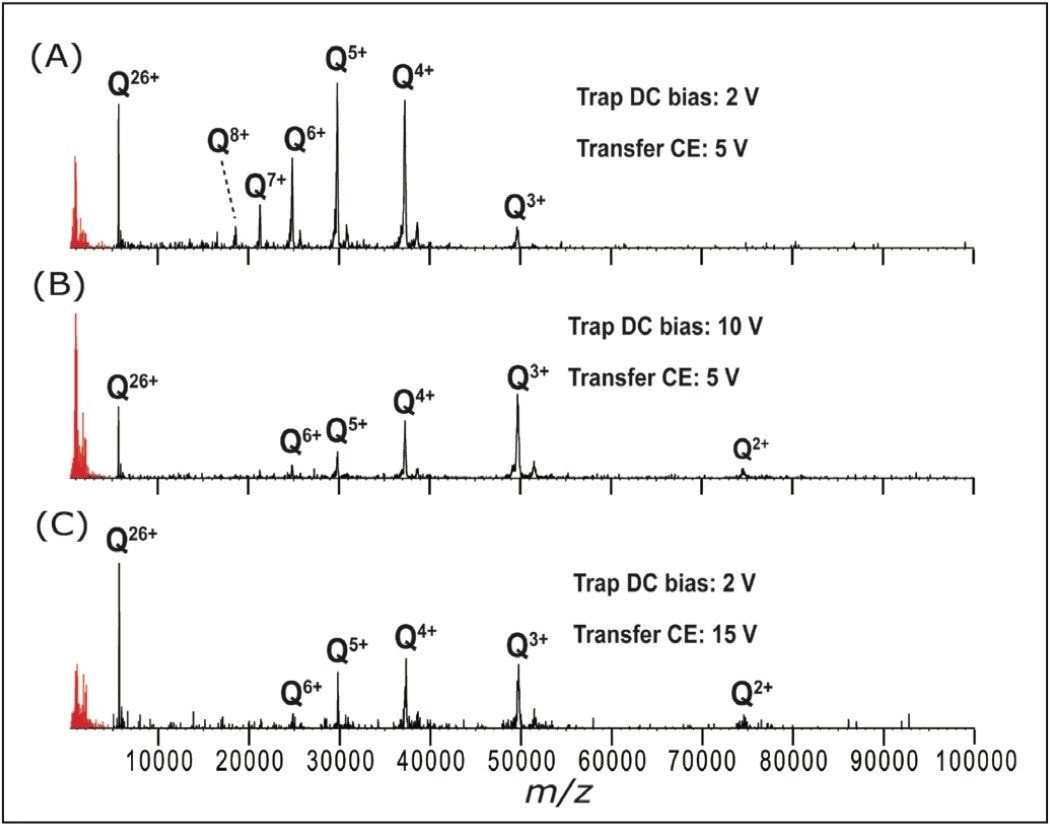
For all proteins, it was necessary to increase the post-ETD acceleration voltages in order to observe the low charge states. Both the trap DC bias and the transfer collision energy proved effective for this (in TOF mode), as shown in Figure 3. ETD fragmentation in the N-terminal region of ADH is visible in the low (600–3200) m/z region in this figure, as reported earlier.6,7
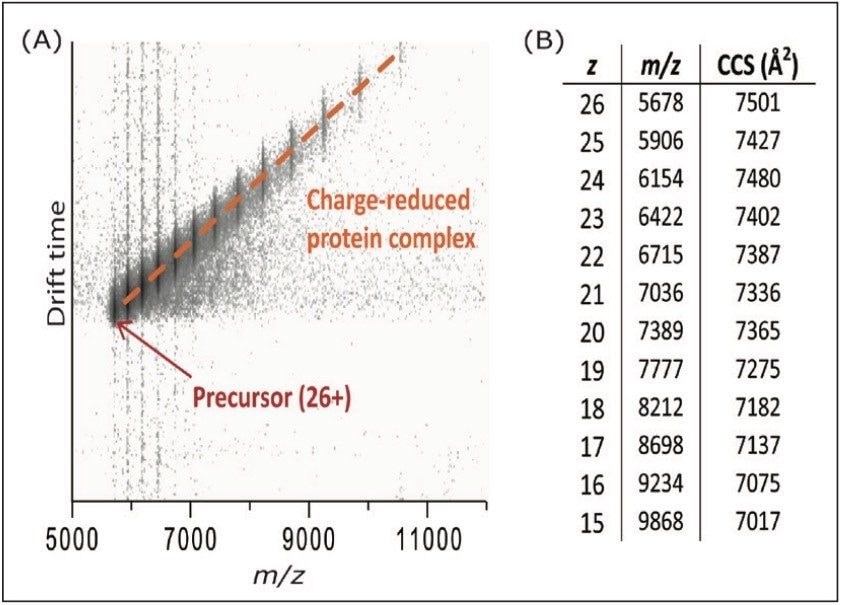
Finally, the IM capabilities of the SYNAPT G2 and SYNAPT G2-S instruments can be used to study the conformation of the charge-reduced complexes, and investigate the relation between the charge and folding state. In order to minimize disturbance of the protein structure, the trap DC bias and transfer CE were kept at 45 V and 2 V, respectively. However, even under these conditions, charge reduction of 26+ ADH down to the 15+ charge state was observed, as shown in Figure 4. The table in Figure 4b shows the calculated collision cross-section (CCS) of each observed charge state. Within the observed range, no major structural changes were observed, other than a gradual decrease in CCS due to reduced Coulombic repulsion. This is consistent with earlier work carried out on a modified ion mobility mass spectrometer.5
Extensive charge reduction of noncovalent protein complexes weighing up to several hundred kDa was demonstrated on unmodified SYNAPT G2 and SYNAPT G2-S instruments using standard ETD reagents. This method was used to partially deconvolute the very complex signal generated by a large, polydisperse assembly, allowing identification of the major species present. Concomitant top-down fragmentation provides sequence information. In combination with ion mobility, the effect of charge state on the global conformation of proteins and protein complexes can be studied.
720005484, November 2015