
This is an Application Brief and does not contain a detailed Experimental section.
This application brief demonstrates the use of UltraPerformance Convergence Chromatography (UPC2) with its orthogonal selectivity to UPLC for use in drug metabolism studies.
UPC2 provides orthogonal and complementary separation compared to UPLC for metabolite detection and identifications.
In drug metabolism, methods are generally developed in the absence of metabolite standards. Typically, most biologically mediated drug metabolism processes make compounds more polar to facilitate their excretion from the body. As a consequence, LC-MS methods must be designed to provide enough gradient space to retain and resolve related unknown compounds (metabolites) which are likely to elute earlier than the parent drug. It is however, difficult to predict how polar these metabolites might be ahead of analysis of real samples. If the parent drug is itself relatively polar, reversed phase retention of polar metabolites is even more challenging. The ACQUITY UPC2 System provides orthogonal selectivity to UPLC since polar metabolites elute later than the parent drug. Coupling UPC2 with ESI-MS analysis can be a powerful and complementary technology in pharmaceutical applications.
Buspirone was incubated in human liver microsomes and analyzed at two time points (0 and 60 min.). Incubations were quenched by protein precipitation and centrifuged. The supernatant was then analyzed using two different chromatographic modes, UPLC and UPC.2 For UPLC, mobile phases used were as follows: A, water + 0.1% formic acid; and B, acetonitrile + 0.1% formic acid. Separations were performed using an ACQUITY UPLC 2.1 x 50 mm BEH C18 1.7 μm Column with a gradient from 5 to 50% B. UPC2 was performed using CO2 with a methanol cosolvent (B) gradient: 0–0.5 min hold at 5% B, 0.5–4 min ramp from 5% to 35% B. UPC2 separations were performed using an ACQUITY UPC2 2.1 x 100 mm BEH 1.7 μm Column. MS information was recorded by coupling either chromatographic system (UPLC or UPC2) to a SYNAPT G2-Si Mass Spectrometer capable of recording HDMSE (low and high energy fragment time-aligned data recorded with ion mobility information). Data was collected using MassLynx Mass Spectrometry Software and was imported into the UNIFI Scientific Information System for data processing.
Figure 1 shows the XICs for the 60-minute incubation for: A – buspirone (intact parent drug); B – buspirone + O (single oxidation metabolites); and C – buspirone + 2O (double oxidation metabolite). In this example we see six oxidative metabolites, with one metabolite eluting after parent (putative N-oxide), while further oxidations result in a rich profile of metabolites, even when analysed with a sub three-min gradient method.
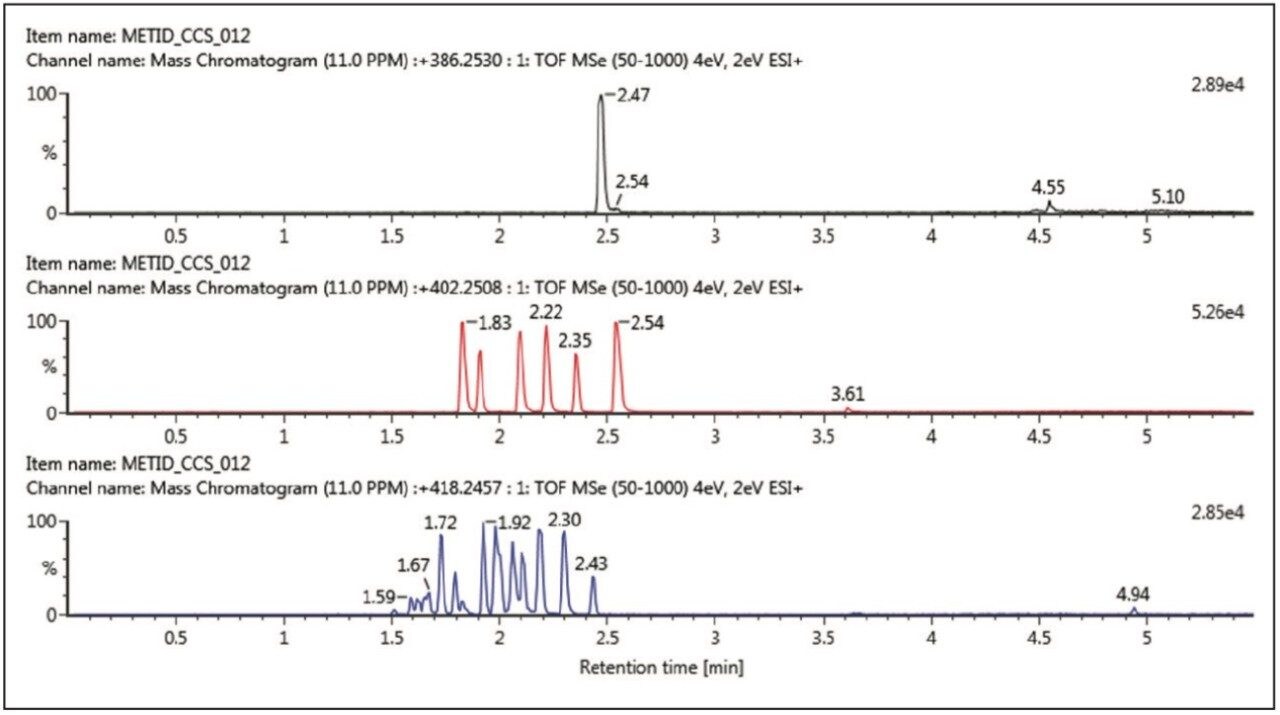
The unique orthogonal selectivity of UPC2 for the same buspirone sample is shown in Figure 2. In this case, we can clearly see all six mono-oxidative species resolved (Figure 2B) with longer retention times than the parent drug. For the doubly oxidized metabolites, good separation is also achieved with a comparable number of detected metabolites. Again, it is achieved with increased retention with respect to the parent drug, buspirone.
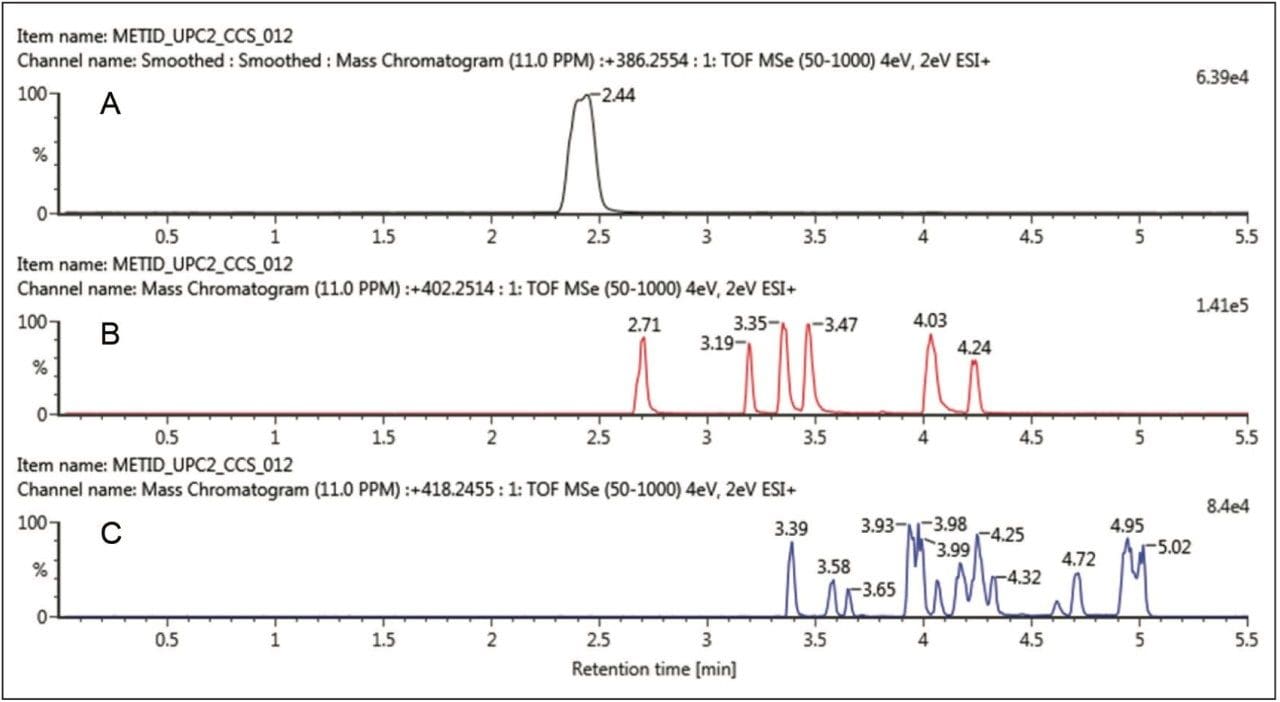
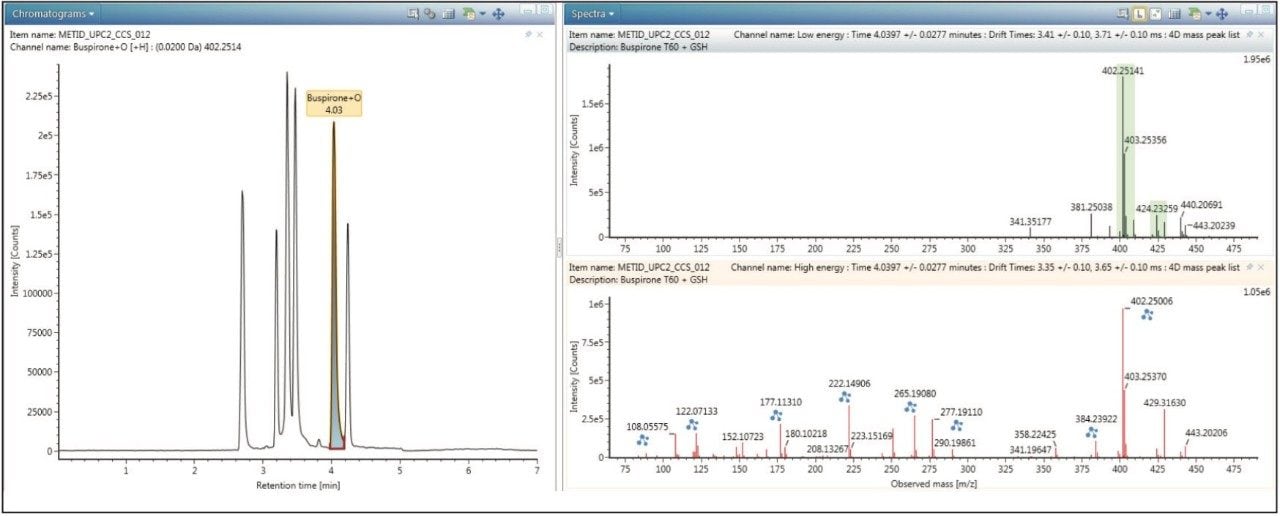
As datasets were obtained on the same MS platform, collecting both ion mobility and MS information, both samples can be interrogated and cross-referenced to each other for maximum coverage and confirmation of the drug metabolism profile. All metabolites can be further processed for additional precursor, fragment ion, and structural information as shown in Figure 3. The spectral data is also drift time resolved (ion mobility) enabling routine and very clean fragmentation information in a convenient screening workflow.
Under both separation modes, UPLC and UPC2 provided useful and fast screening separations for the detection of major metabolites. UPC2 offers a complementary selectivity for screening metabolites in drug metabolism applications. As metabolites are in general more polar than parent drugs, the additional retention observed with UPC2 for metabolites offers an alternative path for challenging analyses beyond traditional reverse phase chemistries. In contrast to conventional normal phase LC separations, UPC2 has high compatibility with MS platforms, which simplifies LC-MS data acquisition and analysis. All data workflows designed for UPLC basic ESI-LC-MS studies on the Waters G2-based QTof platforms are now accessible for UPC2-MS based analyses.
720005130, August 2014