
This application note demonstrates a proper method transfer of a typical HPLC gradient method for abacavir-related compounds. Abacavir (Ziagen) is a nucleoside reverse-transcriptase inhibitor that is used in anti-HIV therapy.
Transferring HPLC gradient methods that use larger volume columns packed with larger particles to smaller volume columns packed with highly-efficient CORTECS 2.7 μm Particles is an easy way to reduce analysis time, solvent and sample consumption, and, ultimately, cost. When transferring the HPLC gradient method avoid compromising the chromatographic separation by properly adjusting the method conditions and selecting the equivalent column chemistry.
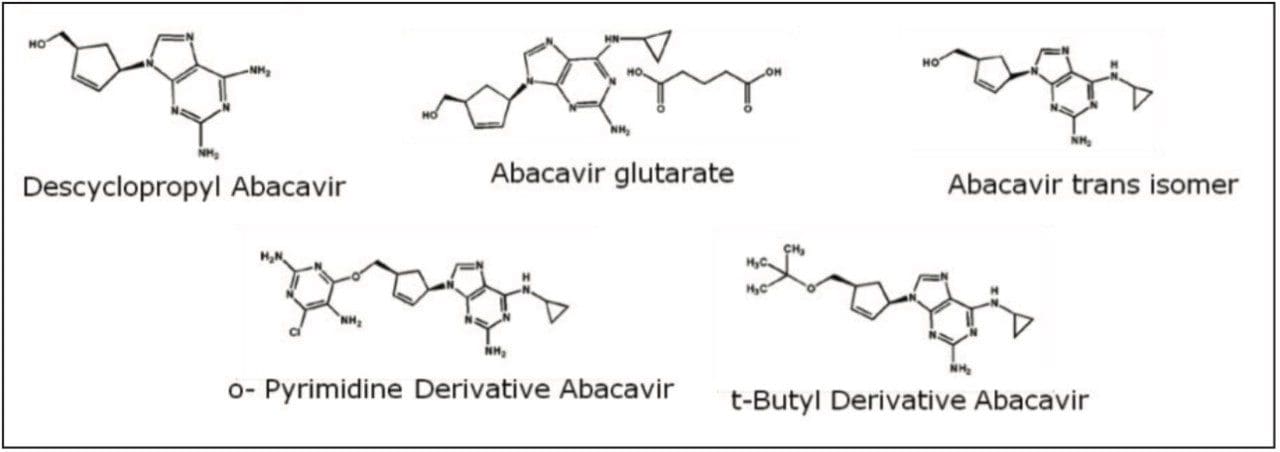
The following application note demonstrates a proper method transfer of a typical HPLC gradient method for abacavir-related compounds. Abacavir (Ziagen) is a nucleoside reverse-transcriptase inhibitor that is used in anti-HIV therapy. The sample is composed of five compounds, four of them related substances of the main component abacavir; all shown in Figure 1. A typical column for this type of assay is a fully porous C18, 5 μm, 4.6 x 150 mm column. The analysis time of this gradient method can be significantly reduced by transferring to a CORTECS C18, 2.7 μm Column, while maintaining the selectivity and the resolution of the peaks of interest.
|
System: |
Alliance HPLC with 2489 TUV detector |
|
Column: |
Fully porous C18, 5 μm, 4.6 x 150 mm |
|
Mobile phase A: |
0.1% trifluoroacetic acid in water |
|
Mobile phase B: |
85% methanol in water |
|
Backpressure: |
1,800 psi |
|
Detection: |
UV at 254 nm |
|
Needle wash: |
90:10 water:acetonitrile |
|
Seal wash: |
80:20 acetonitrile:water |
|
Injection volume: |
8 μL |
|
Time (min) |
Flow rate (mL/min) |
%A |
%B |
|---|---|---|---|
|
Initial |
1.0 |
95 |
5 |
|
23.64 |
1.0 |
70 |
30 |
|
38.39 |
1.0 |
10 |
90 |
|
43.83 |
1.0 |
10 |
90 |
|
44.89 |
1.0 |
95 |
5 |
|
50.00 |
1.0 |
95 |
5 |
|
System: |
Alliance HPLC with 2489 TUV detector |
|
Column: |
CORTECS C18, 2.7 μm, 4.6 x 75 mm (p/n 186007376) |
|
Mobile phase A: |
0.1% trifluoroacetic acid in water |
|
Mobile phase B: |
85% methanol in water |
|
Backpressure: |
4,400 psi |
|
Detection: |
UV at 254 nm |
|
Needle wash: |
90:10 water:acetonitrile |
|
Seal wash: |
80:20 acetonitrile:water |
|
Injection volume: |
4 μL |
|
Sample vial: |
Waters LCGC certified clear glass vial with PTFE/ silicone septa (p/n 186000307C) |
|
Data Management: |
Empower 3 |
|
Time (min) |
Flow rate (mL/min) |
%A |
%B |
|---|---|---|---|
|
Initial |
1.85 |
95 |
5 |
|
6.38 |
1.85 |
70 |
30 |
|
10.37 |
1.85 |
10 |
90 |
|
11.83 |
1.85 |
10 |
90 |
|
12.12 |
1.85 |
95 |
5 |
|
15.00 |
1.85 |
95 |
5 |
Abacavir-related compounds (USP reference standard) 1.0 mg/mL in 100% HPLC-grade water.
Decreasing the particle size increases the number of theoretical plates in a given column length, therefore, shorter length columns can be used and the separation can be maintained. The following equation is used to determine the appropriate column length when changing particle size.
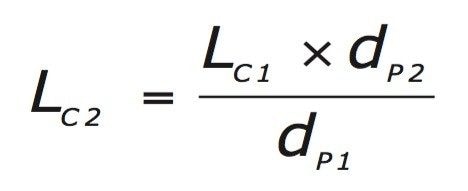
Decreasing column volume requires that the injection volume be adjusted accordingly as described in the following equation.
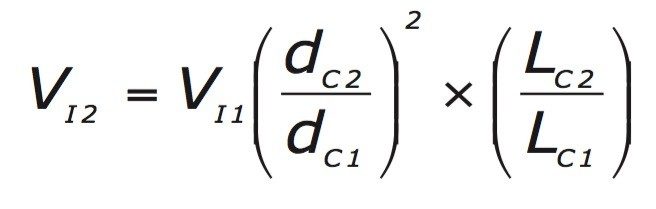
Flow rates must be adjusted as column internal diameter changes to maintain the same linear velocity. The flow rates must also be adjusted in inverse proportion to the change in particle size to maintain the performance; this is done using the following equation.
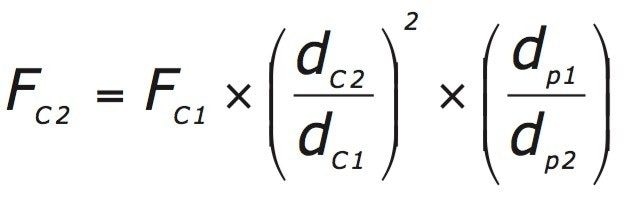
To maintain the same number of column volumes on both columns, the gradient time must be altered to maintain the gradient slope. The gradient time can be adjusted using the following equation.
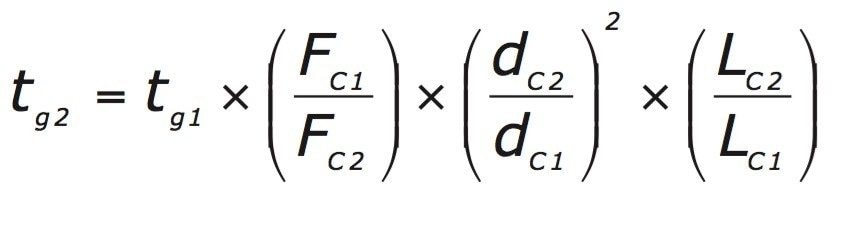
The CORTECS C18, 2.7 μm chemistry was chosen for the transfer; this was based on the fully porous C18, 5 μm column typically used for this type of assay. Transferring to a column packed with 2.7 μm particles requires a 75 mm column length to maintain the L/dp ratio. For this transfer, a 4.6 x 75 mm column configuration was chosen. The change in column length required that the injection volume be adjusted from 8 μL to 4 μL. Since the I.D. of the column was not changed the adjusted flow rate is based on the change in particle size only; the adjusted flow rate was calculated to be 1.85 mL/minute. Adjusting the column configuration and the flow rate requires that each time segment of the gradient also be adjusted to ensure that the separation takes place over the equivalent number of column volumes.
A comparison from the method transfer is shown in Table 1 and in Figure 2. The transferred method using the CORTECS C18, 2.7 μm Column has equivalent selectivity to the method that was performed using a fully porous C18, 5 μm column. Also, the resolution values for two critical peak pairs have been maintained. The 4,400 psi backpressure generated on the CORTECS C18, 2.7 μm, 4.6 x 75 mm Column is well within the 5,000 psi pressure limit of the HPLC instrument. Transferring the HPLC gradient method to the CORTECS C18, 2.7 μm Column reduces the analysis time is by a factor of ~4X and the solvent consumption by ~2X.
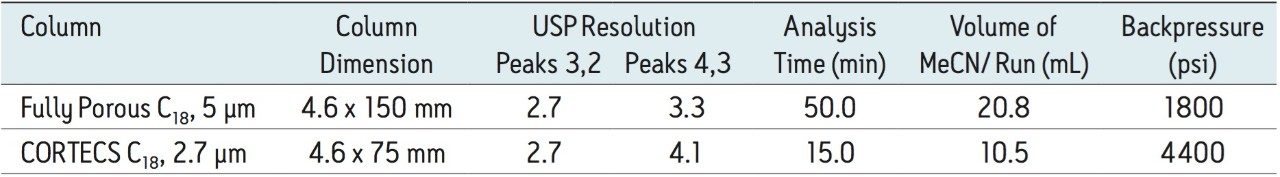
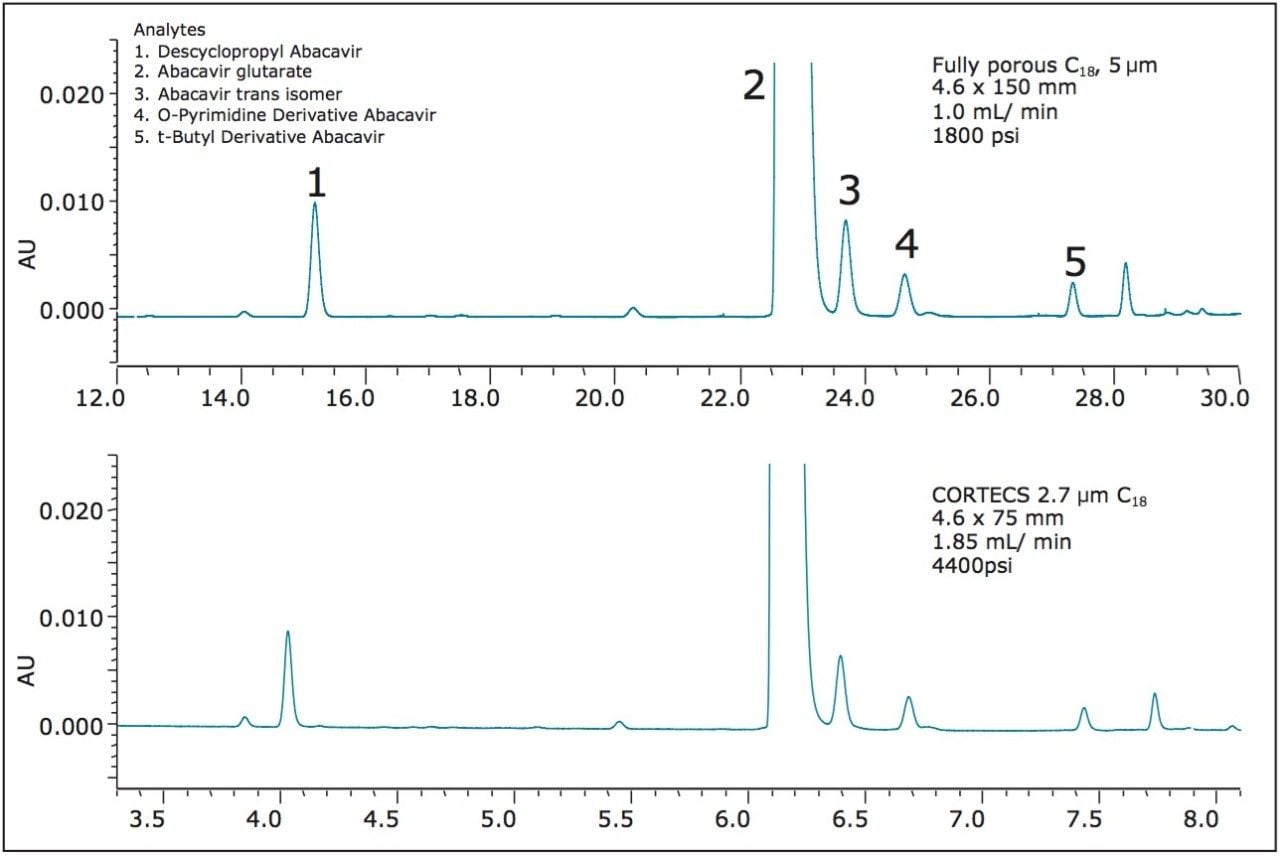
Transferring HPLC gradient methods that use larger volume columns packed with larger particles to smaller volume columns packed with highly efficient CORTECS particles can easily be achieved. A typical HPLC gradient method for the analysis of abacavir-related substances was successfully transferred to demonstrate a ~4X improvement in throughput while maintaining the chromatographic separation. In addition to the time savings, solvent consumption was reduced by ~2X. The backpressure generated when using the CORTECS C18, 2.7 μm, 4.6 x 75 mm Column is well within the limits of the HPLC instrument of <5,000 psi. When transferring HPLC gradient methods to CORTECS 2.7 μm Columns the increase in throughput and the decrease in solvent consumption add up to significant cost savings.
720005104, July 2014