Studies using UPC2 coupled with ultraviolet (UV) detection, mass spectrometry (MS), and evaporative light scattering (ELS) detection for the separation of triacylglycerols in tobacco, corn, sesame, and soybean seed oils are presented in this application note.
UltraPerformance Convergence Chromatography, in combination with sub-2-μm particles and UV, ELS, and MS detection, is a valuable technique for the determination of triacylglycerol composition in a variety of seed oils. Excellent resolution on a single column in as little as 10 minutes serves as an improvement on past generations of SFC instrumentation. This methodology can be used as a tool for rapid characterization and profiling a suite of acylglycerols from different oil sources.
UltraPerformance Convergence Chromatography (UPC2) is a novel technology that applies the performance advantages of UPLC to supercritical fluidchromatography (SFC). Combining the use of supercritical CO2 with sub-2-μm particle columns, UPC2 represents an analysis technique that is orthogonal to reversed-phase LC and can be used to solve many troublesome separations that challenge conventional LC or GC analyses. It also generates less solvent waste as compared to liquid chromatography. These benefits have led to interest in applying this technology to various industrial analytical areas. The established UPC2-MS approach has potential application in lipidomics as a complementary method alongside LC-MS and GC-MS, as it can separate both polar and non-polar lipids, and in many cases does not require derivatization of lipids to improve detection limits and peak shape. Studies using UPC2 coupled with ultraviolet (UV) detection, mass spectrometry (MS), and evaporative light scattering (ELS) detection for the separation of triacylglycerols in tobacco, corn, sesame, and soybean seed oils are presented in this application note. A single unendcapped C18 column with acetonitrile-modified CO2 was used for the separation of all seed oils.
Tobacco seed oil was obtained from R.J. Reynolds Tobacco Co. (Winston-Salem, NC); soybean oil, corn oil, and sesame seed oil were obtained from Sigma Aldrich (St. Louis, MO). 5% of the different oils were dissolved in dichloromethane/methanol (1/1) for UV and ELS and 0.1% for the Xevo G2 Q-Tof MS.
|
System: |
ACQUITY UPC2 |
|
Column: |
ACQUITY UPC2 HSS C18 SB, 1.8 μm, 3.0 x 150 mm |
|
ABPR: |
1500 psi |
|
Column temperature: |
25 °C |
|
Injection volume: |
2-8 μL (UV and ELSD), 0.5 μL (MS) |
|
Sample solvent: |
Dichloromethane/methanol (1/1) |
|
Flow rate: |
1-2 mL/min |
|
Mobile phase A: |
Compressed CO2 |
|
Mobile phase B: |
Acetonitrile or 90/10 acetonitrile/MeOH |
|
Make up solvent: |
IPA for ELSD and MeOH in 10 mM ammonium acetate for MS |
|
Make up flow rate: |
0.2 mL/min |
|
Gradient: |
A- 98/2 to 80/20 in 18 min for ELSD/UV and 18 min for MS B- 90/10 to 50/50 in 10 minutes for MS |
|
Detectors: |
ACQUITY UPC2 PDA 200-400 nm, Ref. 400-500 nm ACQUITY ELS Nebulizer: Cooling, drift tube: 50 °C, Gas pressure: 40 psi and Gain 10 |
|
MS: |
Xevo G2 Q-Tof |
|
Ionization mode: |
ESI positive |
|
Capillary voltage: |
3.0 kV |
|
Cone voltage: |
30 V |
|
Source temperature: |
150 °C |
|
Desolvation temp.: |
500 °C |
|
Cone gas flow: |
10 L/h |
|
Desolvation gas flow: |
600 L/h |
|
Acquisition range: |
40 to 1200 m/z |
From the perspective of current applied lipid research, UPC2 is a complementary and perhaps preferred technique to gas chromatography (GC) and high performance liquid chromatography (HPLC) for metabolic profiling of lipids.1 The use of UPC2-MS allows for high throughput and exhaustive analysis of diverse lipids, leading to potential application in lipidomics. Currently, there is much interest in rapid characterization of triacylglycerols (TAGs). TAGs are natural compounds produced by the esterification of glycerol with fatty acids. In humans, TAGs serve as a source of energy stored in fat tissues and they form a thermal and mechanical protective layer around important organs.2 Furthermore, TAGs are the source of essential fatty acids such as linoleic and linolenic acids.
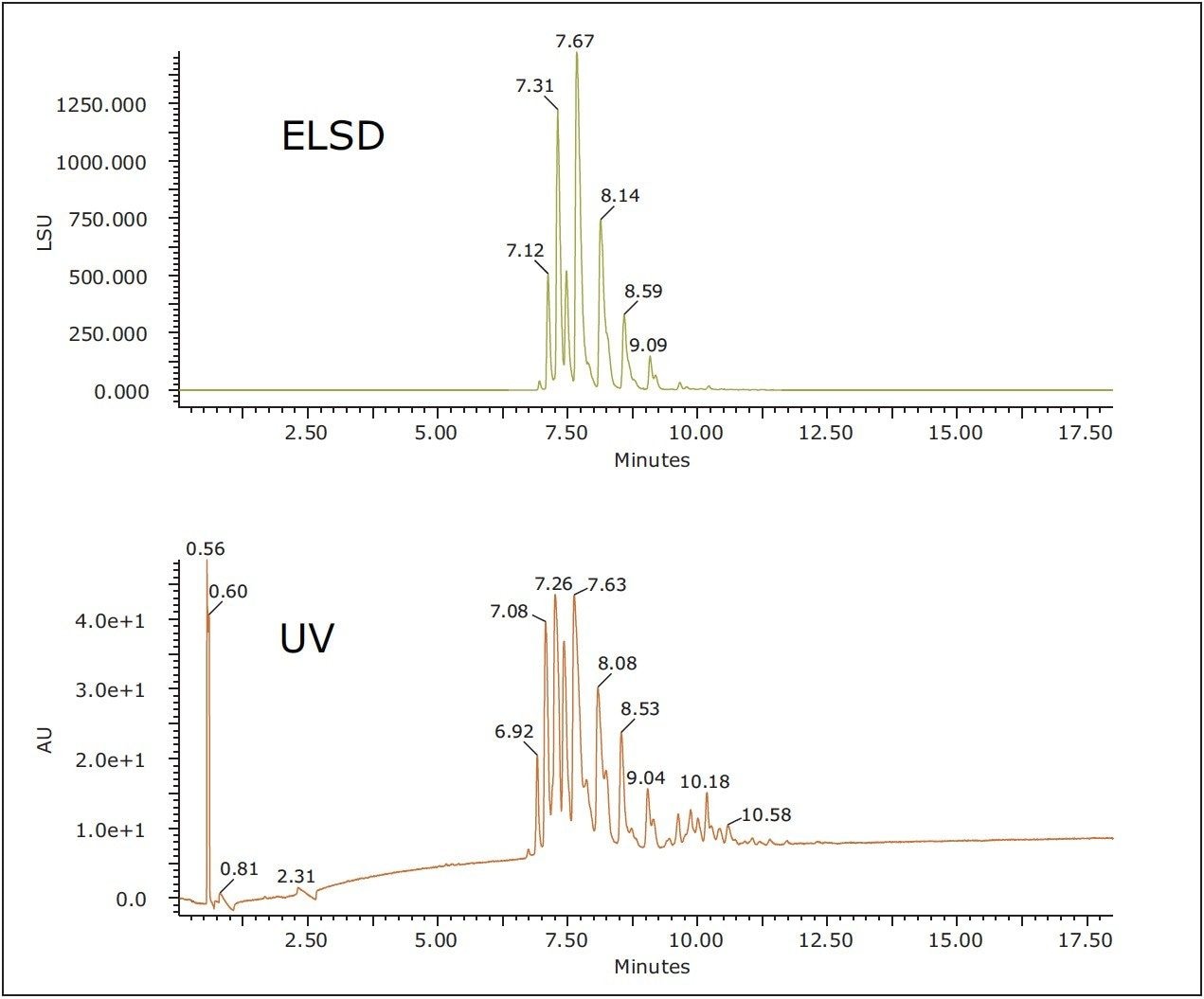
Figure 1 shows UV, ELSD, and MS chromatograms for the separation of TAGs in soybean oil. All of the effluent first passes through the PDA flow cell and then to the back pressure regulator and the ELSD using the PEEK ELSD splitter kit (205001048). Each gradient separation was performed at 25 °C with an ACQUITY UPC2 HSS C18 SB Column and a mobile phase of acetonitrile-modified CO2. Near baseline peak resolution was observed on a single ACQUITY UPC2 HSS C18 SB, 1.8 μm, 3.0 x 150 mm Column in approximately 16 minutes. Baseline stability was excellent, even under gradient conditions, demonstrating the stability of the system and allowing reproducible detection and possible quantitation of lower level peaks in the samples.
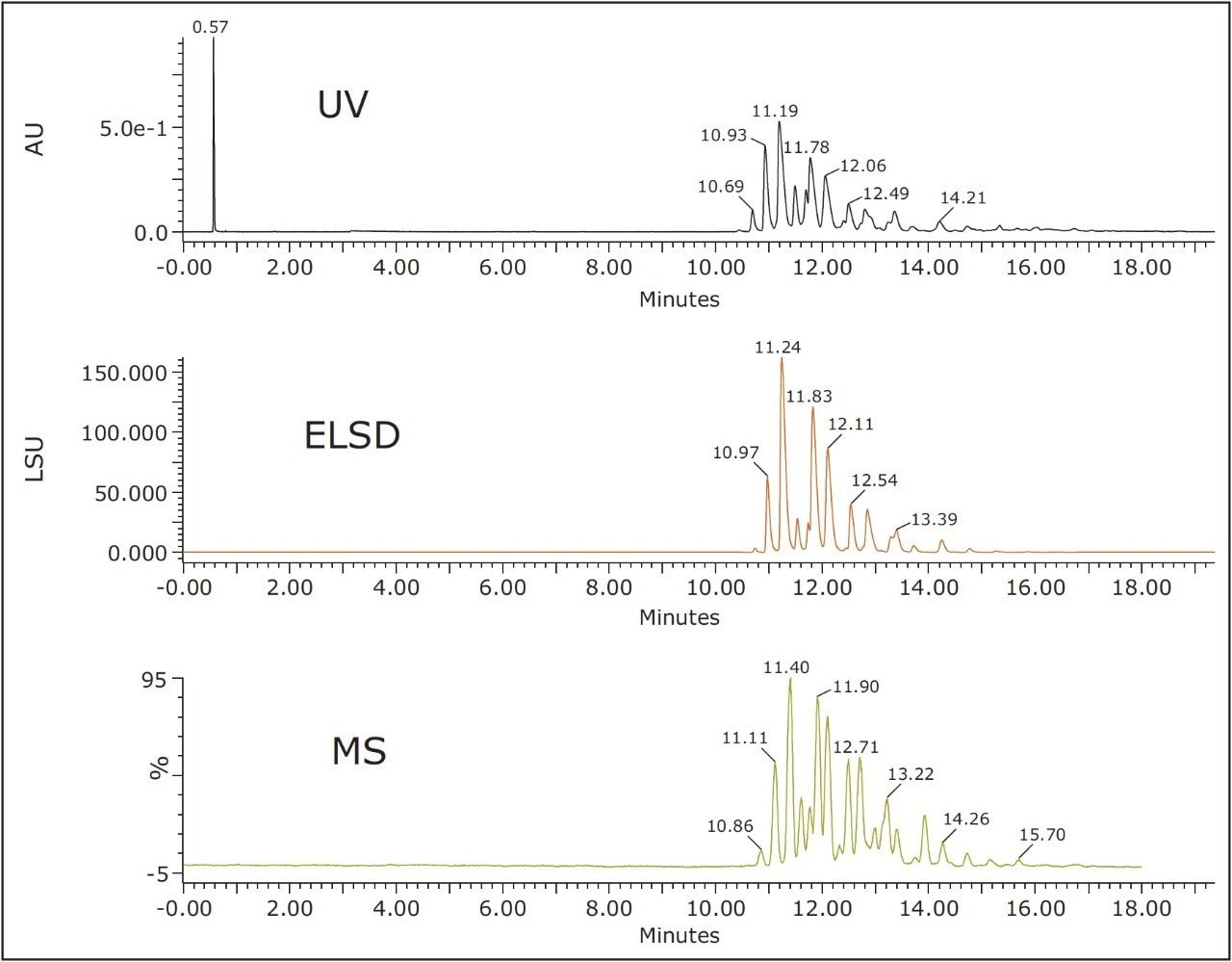
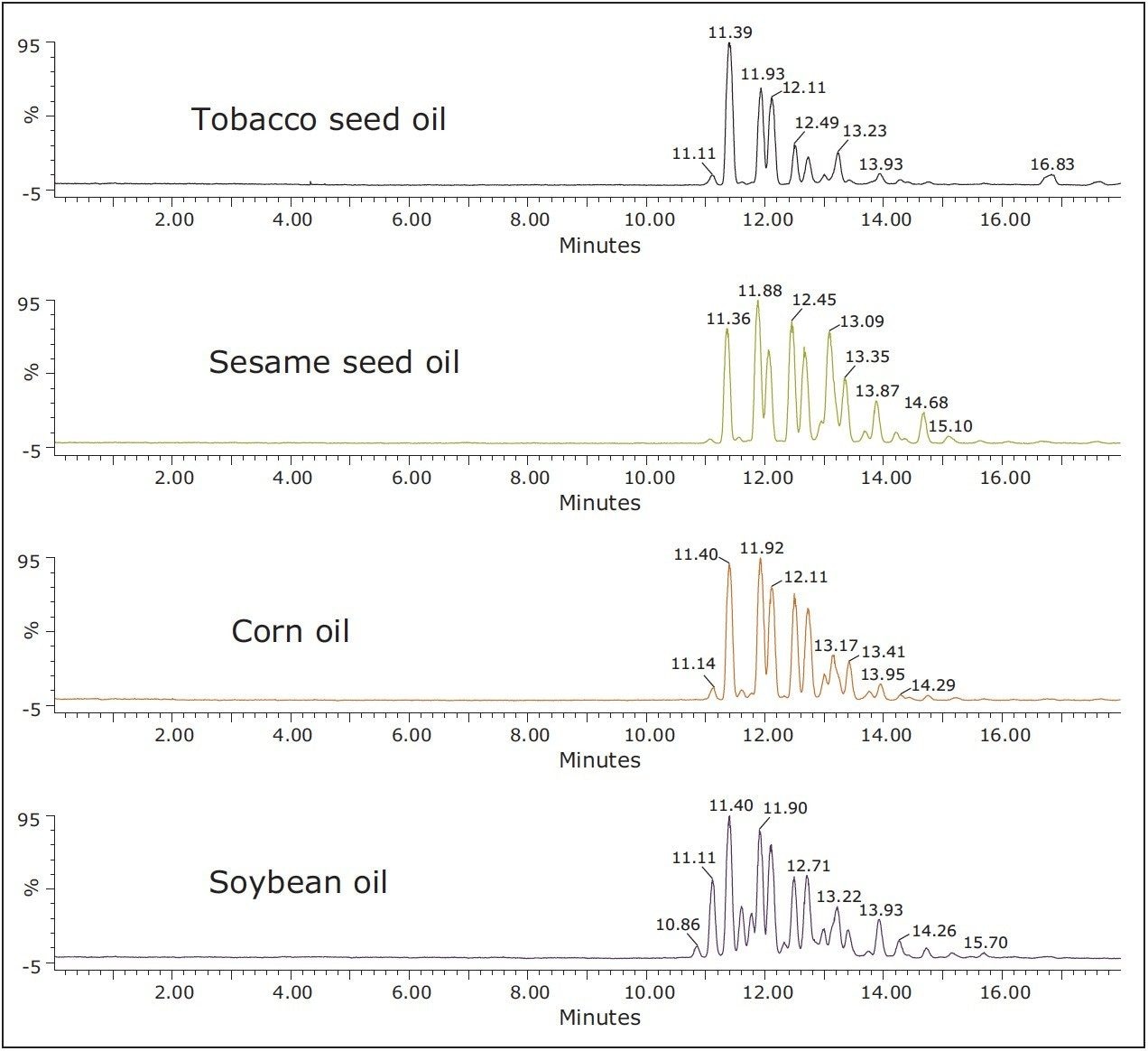
The conditions from Figure 1 were used to separate and profile TAGs in tobacco seed oil, soybean oil, corn oil, and sesame seed oil. Data were acquired in UPC2-MSE mode, an unbiased Tof acquisition method in which the mass spectrometer switches between low and high energy on alternate scans for structural elucidation and identification. In all cases, distinct profiles and excellent separations were obtained for all oil types when using UPC2 with both MS and ELS detection. Figure 2 shows the UPC2-MS separation and detection of different TAGs in different oils using UPC2-MSE. TAGs were identified using accurate mass spectra collected by QTof MS with MSE and Waters TransOmics Informatics. In positive ion mode MSE low energy, TAGs produce intact ammonium adduct [M+NH4]+ precursor exact mass when ammonium acetate is present in the make-up solvent. In MSE high energy, abundant fragment ions are produced corresponding to the neutral loss of one of the sn-1, sn-2, or sn-3 fatty acids plus ammonia.
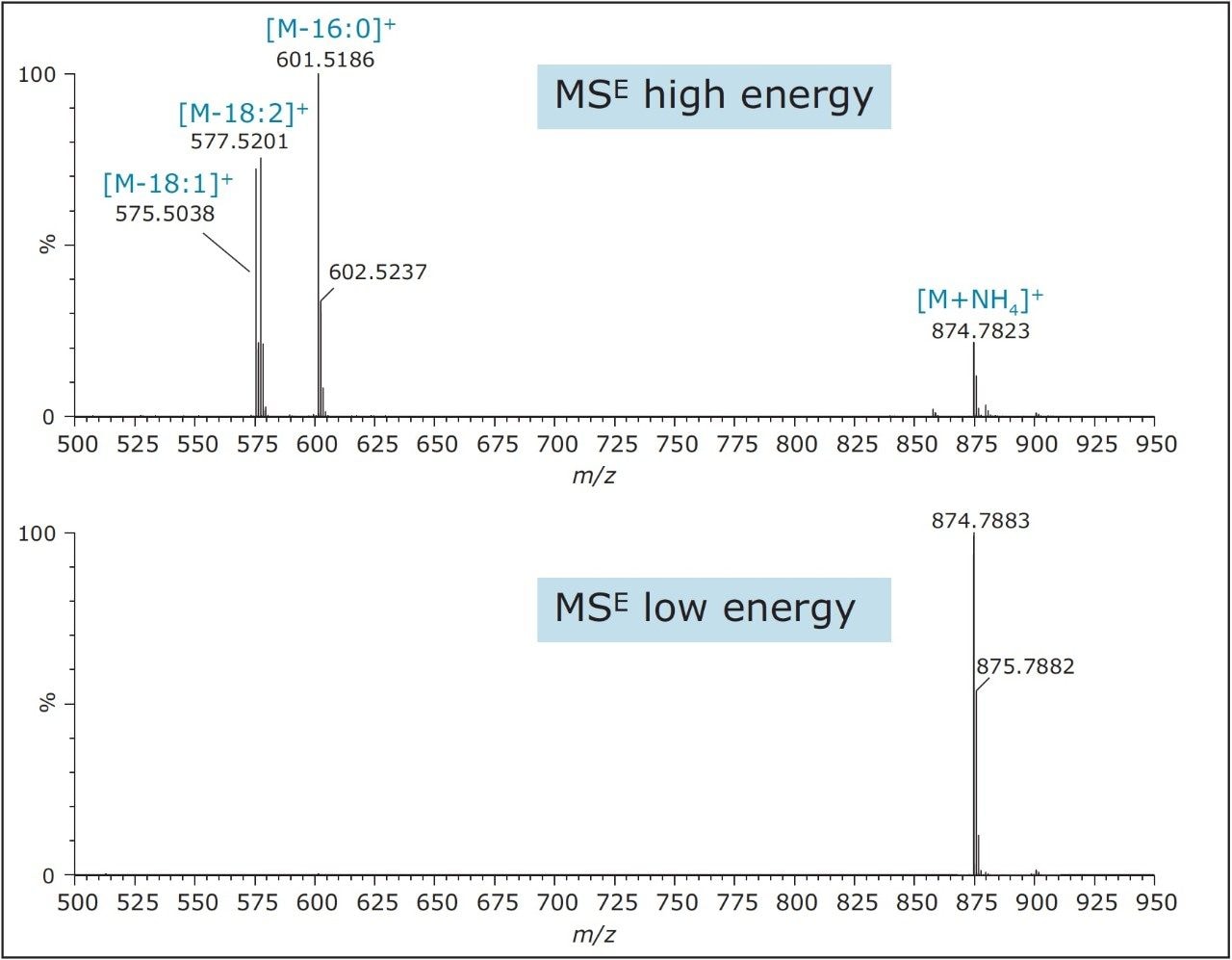
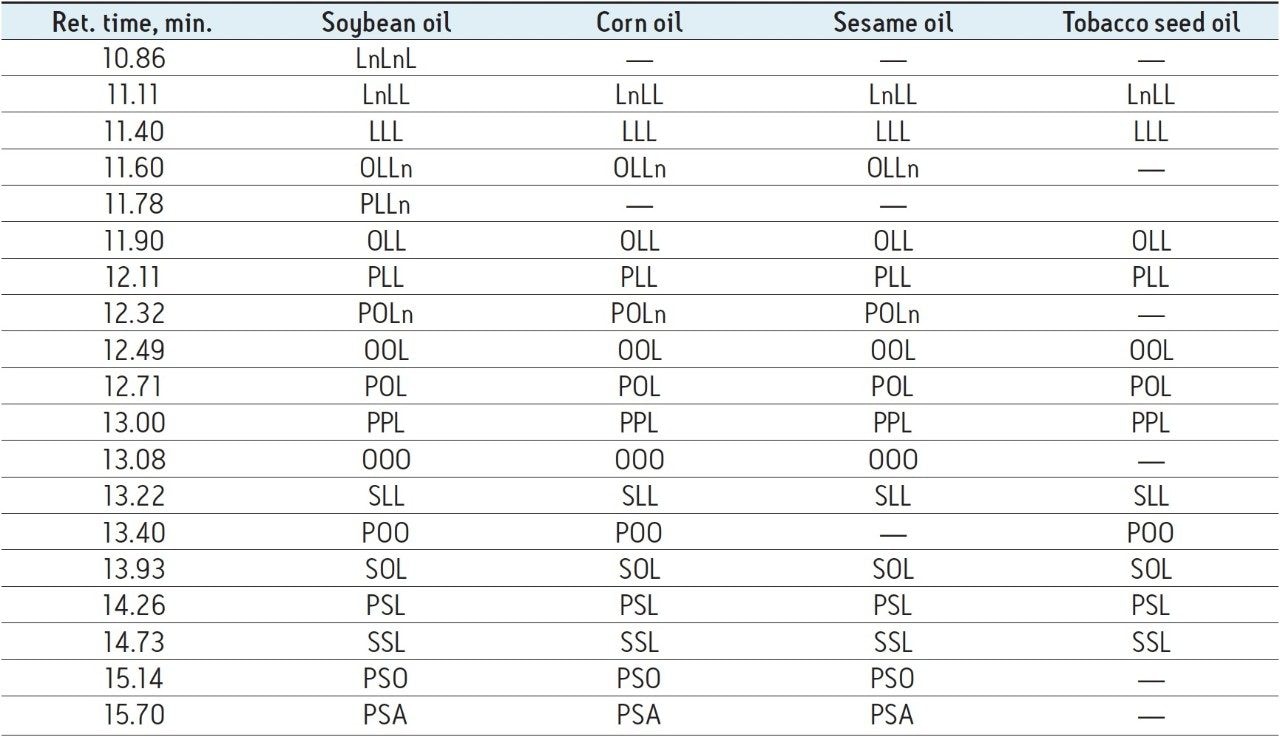
For example, Figure 3 shows the ion at m/z 874.7823 corresponding to 52:3 TAG (calculated fatty acid carbon atom: total number of double bonds), which can be identified as POL due to the presence of abundant fragment ions at m/z 575.5038, 577.5201, and 601.5186, corresponding to the neutral loss of fatty acyl groups P, O, and L plus ammonia, respectively.4 Table 1 shows a list of all identified TAGs in the different oils based on the low energy precursor exact mass and corresponding high energy fragment ions.
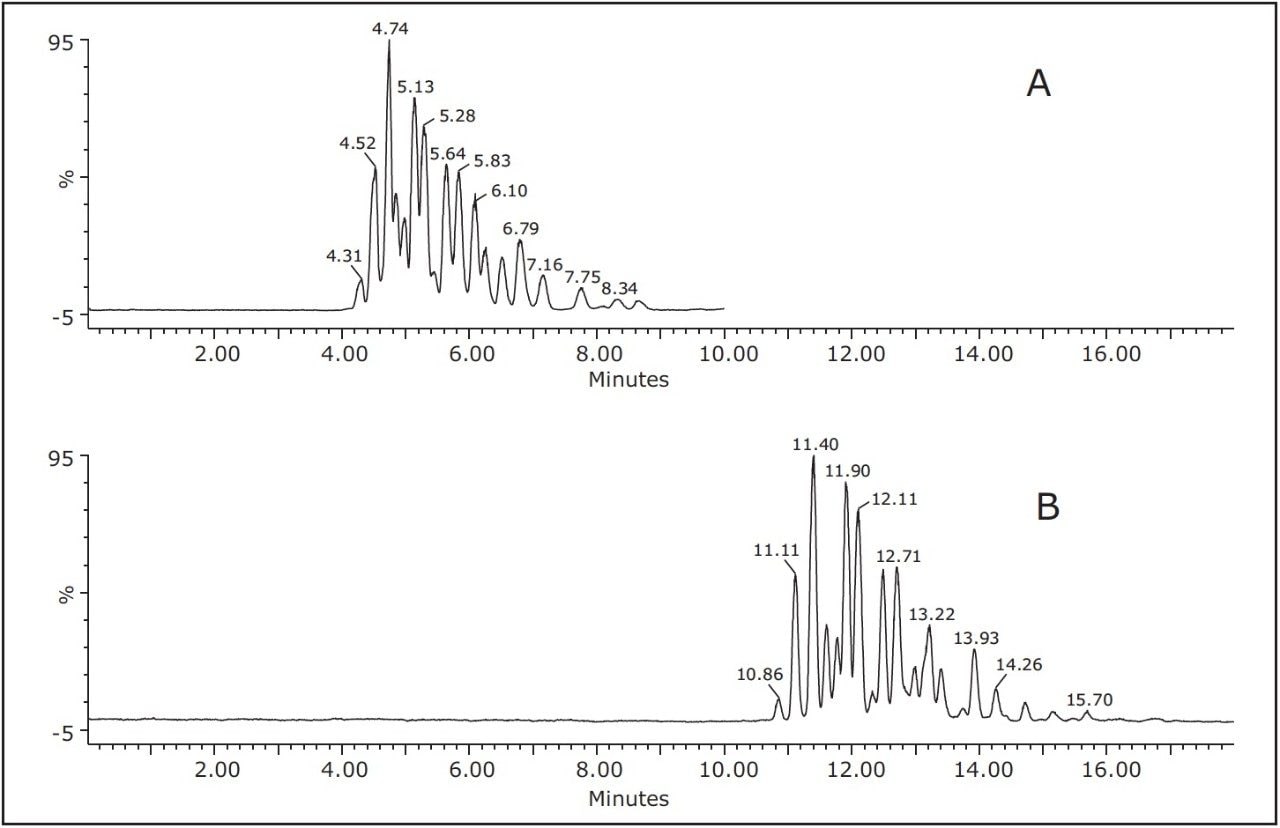
In an attempt to reduce the analysis time of the TAGs, a faster gradient elution was used. Figure 4 shows the separation of soybean oil using two different gradient profiles with acetonitrile as a modifier. With the faster gradient elution (Figure 4A), all the components were eluted in less than nine minutes. With slower gradient elution (Figure 4B), all components eluted in less than 16 minutes. Little resolution was lost when the faster gradient was used, thus increasing the throughput of the UPC2-MS method.
Also, a mobile phase modifier of acetonitrile and methanol (9:1) was tested under the same chromatographic conditions.
Overall retention time decreased approximately 5 minutes with minimal loss in peak resolution (Figure 5). This data indicates that changing the gradient profile, flow rate, and mobile phase modifier can be performed to optimize the separation based on the type of TAG analysis required. All of these parameters are compatible with MS detection, thus allowing positive identification of all TAG species in oils.
UltraPerformance Convergence Chromatography, in combination with sub-2-μm particles and UV, ELS, and MS detection, is a valuable technique for the determination of triacylglycerol composition in a variety of seed oils. Excellent resolution on a single column in as little as 10 minutes serves as an improvement on past generations of SFC instrumentation. This methodology can be used as a tool for rapid characterization and profiling a suite of acylglycerols from different oil sources.
720004871, January 2014