
For forensic toxicology use only.
This application note details a strategy for the successful extraction and analysis of representatives of several different classes of synthetic cannabinoids from urine samples for forensic toxicology. A panel of 22 synthetic cannabinoid drugs and metabolites were extracted from urine and analyzed by UPLC-MS/MS. The use of Oasis HLB μElution plates enabled the simultaneous extraction of acidic, basic and neutral compounds, ensuring that a wide variety of compounds and metabolites could be analyzed. Separation using the CORTECS UPLC C18 Column enabled the analysis of all compounds in a short analysis time with baseline resolution of critical isobaric pairs.
Synthetic cannabinoids, often referred to or marketed as “Spice” compounds, constitute a growing challenge for law enforcement agencies and forensic laboratories. These designer drugs mimic the psychoactive effects of natural cannabinoids. Often labeled as “not for human consumption” and marketed as a legal alternative to natural cannabis, their popularity and use have risen substantially in the last several years.1,2 While recent legislation has banned some of these compounds, minor modifications to existing structures have resulted in a proliferation of substances designed to circumvent existing laws. This application note details a strategy for the successful extraction and analysis of representatives of several different classes of synthetic cannabinoids from urine samples for forensic toxicology. Twenty-two synthetic cannabinoids and metabolites were extracted from urine using Waters Oasis HLB μElution plates. Analytical separation was achieved using Waters’ newly developed solid-core particle UPLC Column (CORTECS) with optimally packed 1.6 μm particles, resulting in excellent chromatographic performance and separation efficiency. Calibration curves for all compounds were linear from 1-100 ng/mL. Quantitative results from quality control samples were accurate and precise across the calibration range. The analysis of several different classes of these drugs and metabolites should render this method applicable to newly developed related compounds with little, if any, modification necessary.
|
LC System: |
ACQUITY UPLC I-Class |
|
Column: |
CORTECS UPLC C18, 1.6 μm, 2.1 x 100 mm (p/n 186007095) |
|
Column temp.: |
30 °C |
|
Injection volume: |
5 μL |
|
Flow rate: |
0.6 mL/min. |
|
Mobile phase A: |
0.1% formic acid in MilliQ water |
|
Mobile phase B: |
0.1% formic acid in ACN |
|
Gradient: |
Initial conditions started at 30% B. The %B was increased to 50% over 2 minutes, held at 50% B for 1 minute, increased to 90% B over 4 minutes and then returned to 30% over 0.2 minutes. The system was allowed to re-equilibrate for 1.3 min. The entire cycle time was 8.5 min. |
|
Vials/plates: |
96-well collection plates with 700 μL deactivated glass inserts (p/n 186000349DV) |
|
MS system: |
Xevo TQD Mass Spectrometer |
|
Ionization mode: |
ESI Positive |
|
Acquisition mode: |
MRM (See Table 1 for transitions) |
|
Capillary voltage: |
1 kV |
|
Collision energy (eV): |
Optimized for individual compounds (See Table 1) |
|
Cone voltage (V): |
Optimized for individual compounds (See Table 1) |
All data were acquired and analyzed using Waters MassLynx Software v.4.1 and quantitated using TargetLynx Software. MS conditions were optimized using IntelliStart.
AM2233, JWH-015, RCS-4, JWH-203, RCS-8, JWH-210, JWH-073, and JWH-018 were purchased from Cerilliant (Round Rock, TX). All other compounds and metabolites were purchased from Cayman Chemical (Ann Arbor, MI)
Individual stocks (1 mg/mL) were prepared in methanol, DMSO, or 50:50 DMSO:methanol. A combined stock solution of all compounds (10 μg/mL) was prepared in methanol. Working solutions were prepared daily by spiking standards into matrix (urine) and performing serial dilutions to achieve the desired concentrations. Calibrator concentrations ranged from 0.5-100.0 ng/mL for all analytes. Quality control samples were prepared at 2.5, 7.5, and 75.0 ng/mL, in urine.
The 22 compounds analyzed are listed in Table 1 and constitute a panel that includes various classes of forensically relevant synthetic cannabinoids. These include adamantoylindoles (AM 1248 and AKB48), napthoylindoles (JWH 022), phenylacetyl indoles (RCS-4 and RCS-8), and tetramethylcyclopropylindoles (UR-144 and XLR11). Major metabolites of JWH-073 and JWH-018 were also included, as some of these compounds are structural isomers with identical mass spectral fragments that require adequate chromatographic separation for accurate quantitation.
Samples were extracted using Oasis HLB μElution plates (p/n 186001828BA). 0.5 mL of 0.8 M potassium phosphate buffer (pH 7.0) was added to 1.0 mL of urine, followed by 10 μL of β-glucuronidase (>140 IU/mL; Roche). After incubation at 40 °C for 1 hr, 1.5 mL of 4% H3PO4 was added to all samples. 600 μL of the final prepared sample (equivalent to 200 μL urine) was then extracted using the Oasis HLB μElution plate. All wells were conditioned with 200 μL methanol (MeOH) and 200 μL H2O. 600 μL of the hydrolyzed, pretreated urine sample was then loaded in each well. All wells were washed with 200 μL water and 200 μL 50:50 H2O:MeOH. Samples were eluted with 2 x 25 μL aliquots of 60:40 ACN:isopropanol (IPA).
All samples were diluted with 75 μL H2O and 5 μL was injected onto the UPLC System. Analyte recovery was calculated according to the following equation:
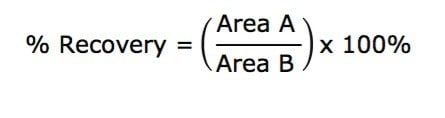
Where A equals the peak area of an extracted sample and B equals the peak area of an extracted matrix sample in which the compounds were added post-extraction.
Matrix effects were calculated according to the following equation:

The peak area in the presence of matrix refers to the peak area of an extracted matrix sample in which the compounds were added post-extraction. The peak area in the absence of matrix refers to analytes in a neat solvent solution.
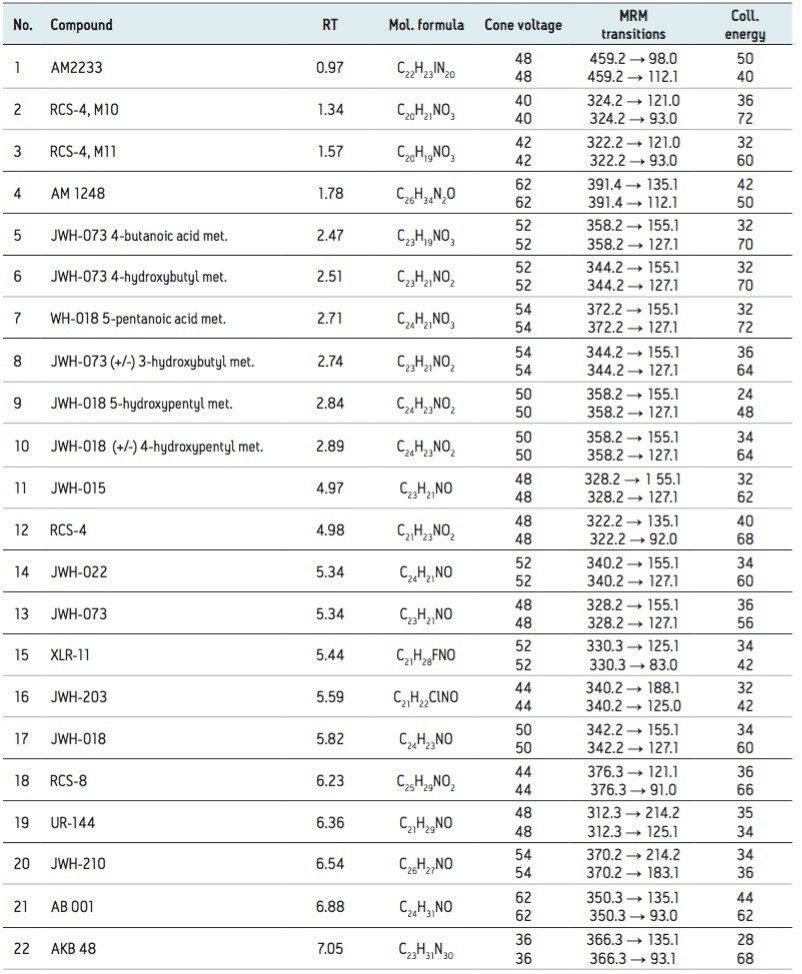
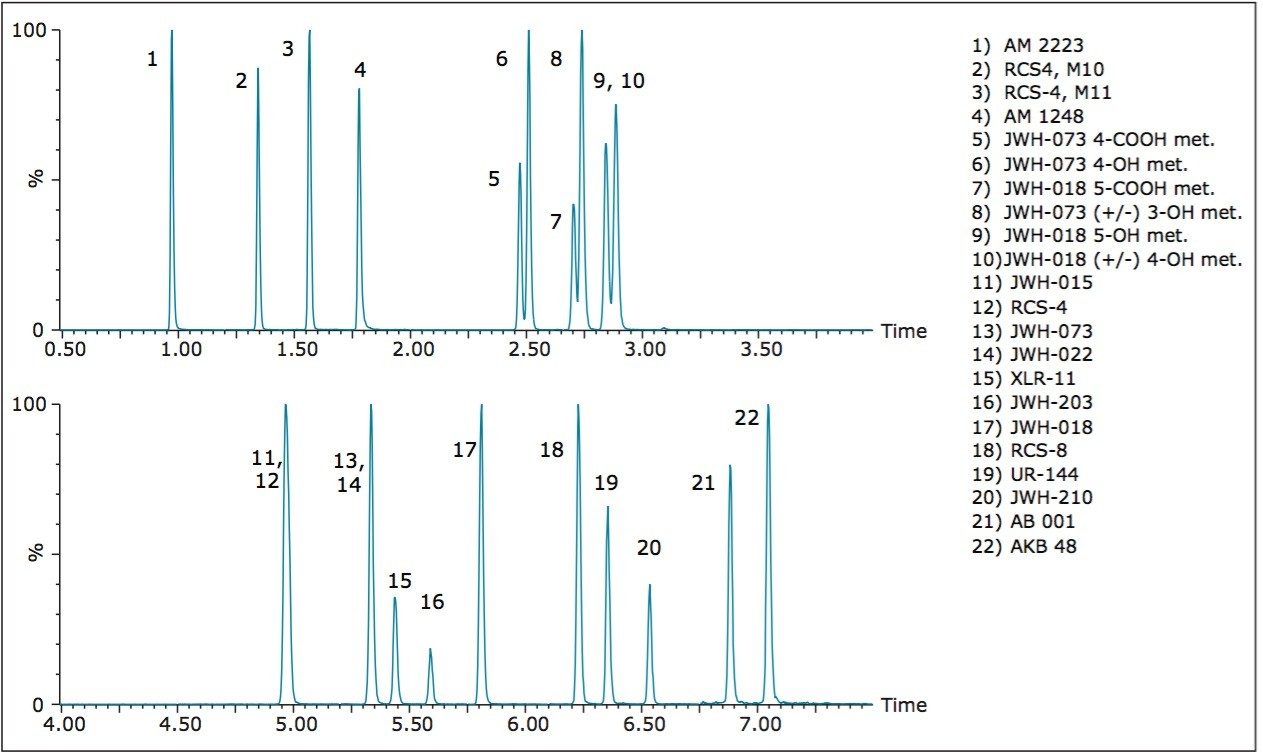
The design of the solid-core CORTECS particle combined with optimal packing in the column results in excellent chromatographic performance. A representative chromatogram of all compounds from a 20 ng/mL calibration standard is shown in Figure 1. Peak assignments are listed in Table 1. Using a CORTECS UPLC C18 Column (2.1 x 100 mm; 1.6 μm), all analytes were analyzed within 7.5 minutes with a total cycle time of 8.5 minutes. Peak shape was excellent for all compounds, with no significant tailing or asymmetries, and all peak widths were under 3 seconds at 5% peak height. Peaks 9 and 10, an isobaric pair of metabolites with identical precursor and product ions, were nearly baseline resolved, with a calculated resolution of 1.04, enabling unambiguous identification and quantitation that would not be possible if the two compounds co-eluted. When the same mix of compounds was analyzed on an ACQUITY UPLC BEH C18 Column (also 2.1 x 100 mm), adequate separation was not achieved for these two compounds. Co elution of compound pairs 5 and 6 and 7 and 8 were also seen on the BEH C18 Column. Figure 2 highlights the improvements in chromatography seen for compounds 5-10 when using the CORTECS C18 Columns. the BEH C18 Column. A more thorough comparison revealed that peak widths on the CORTECS Columns were reduced and peak capacities were improved compared with two analogous, fully porous UPLC Columns of matched dimensions (BEH C18 and HSS T3). On average, peak widths on the CORTECS Columns were 12% and 23% narrower, respectively, than those on the BEH and HSS T3 columns. This improvement in performance would also be expected to be seen over other commercially available fully porous UHPLC columns of similar particle sizes as a result of the performance advantages of the solid core particles and the optimal packing of the CORTECS Columns.
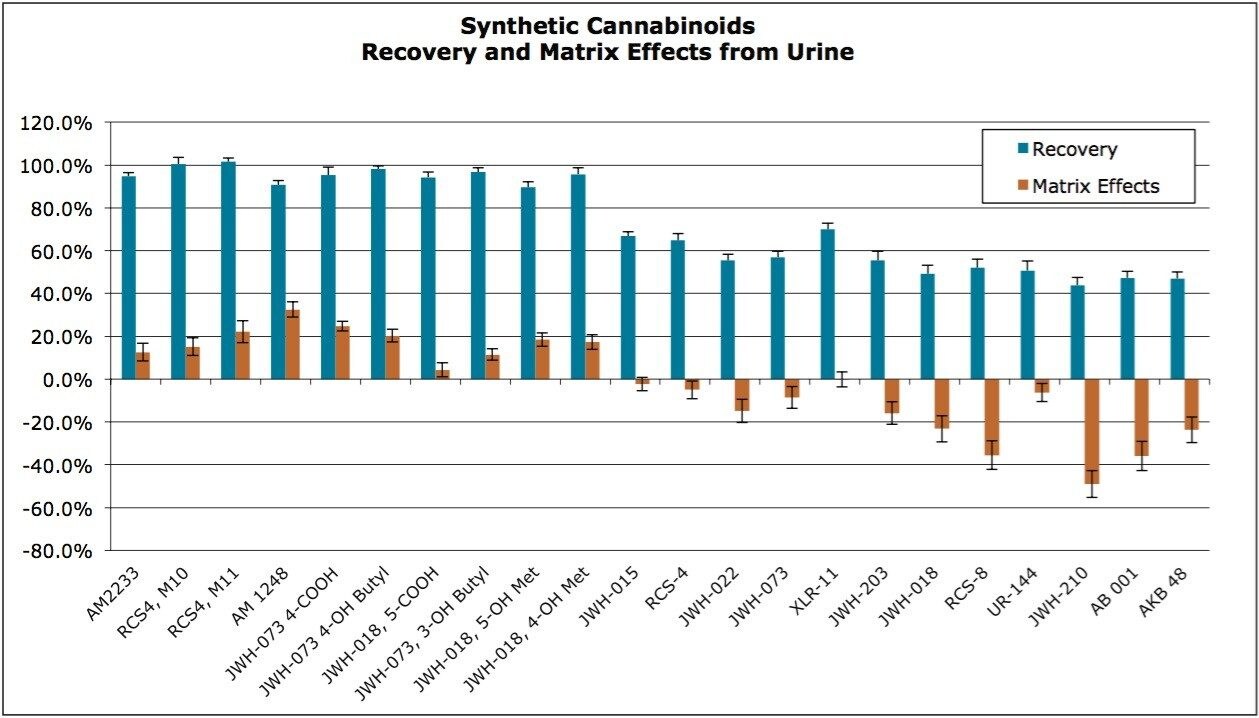
The synthetic cannabinoids and metabolites in this application include compounds that are neutral, acidic and basic. Use of the Oasis HLB sorbent enabled the simultaneous extraction of all of the compounds and metabolites tested, regardless of their functionality. Recoveries and matrix effects were calculated according to the equations described in the experimental section and the results are shown in Figure 3. Recoveries ranged from 44-102% with an average of 74%. Matrix effects ranged from -49% (ion suppression) to 32% (enhancement), although most were less than 20%. Even in instances in which recovery was comparatively low, there was more than adequate sensitivity for the purposes of this assay.
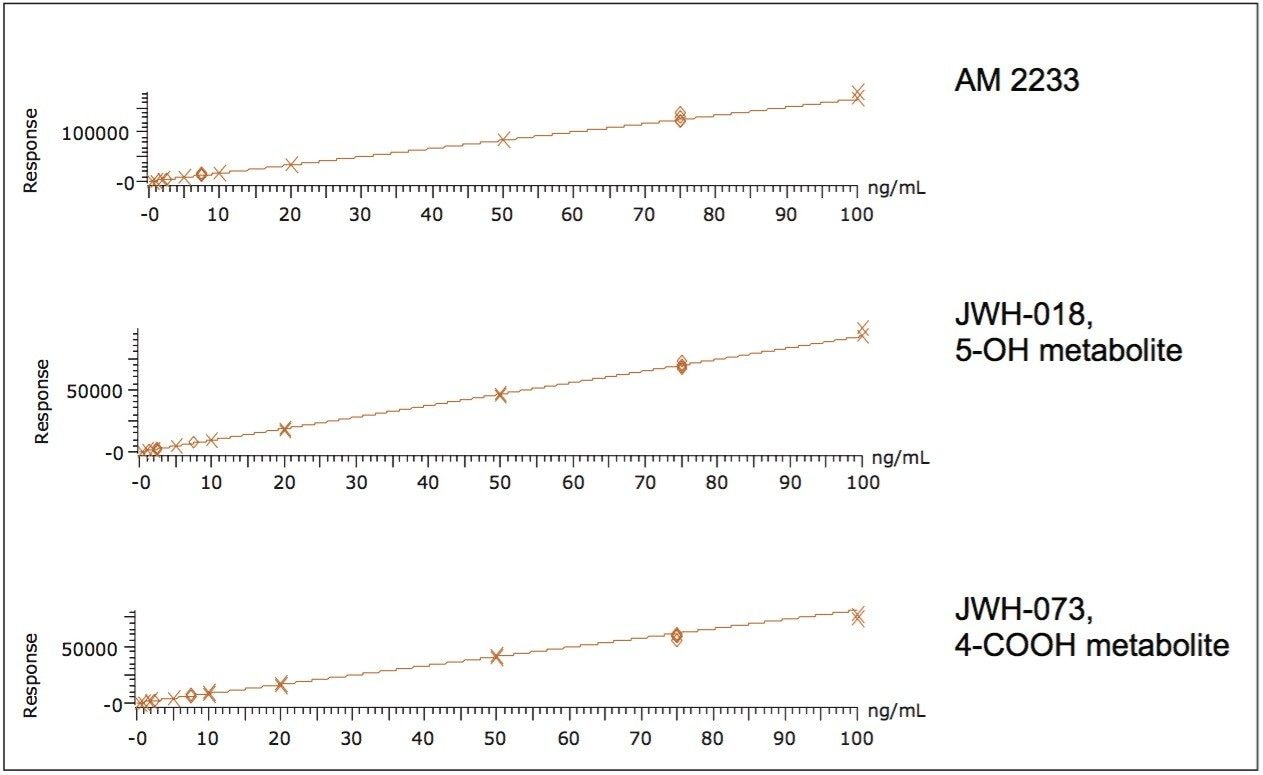
In order to assess linearity and analytical sensitivity, calibration curves were extracted at concentrations ranging from 0.5-100.0 ng/mL for all components. Figure 4 shows representative calibration curves from acidic, neutral and basic compounds (JWH-073 4-COOH metabolite, JWH-018 5-OH metabolite, and AM2233, respectively). These three example curves demonstrate that the Oasis HLB μElution plate can be used to extract a diverse range of synthetic cannabinoids and metabolites alike with a high degree of accuracy. This is important as both the terminal hydroxylated and carboxylic acid metabolites of JWH-018 and JWH-073 have been shown to be present in substantial amounts in human urine.3,4 Quality control samples (N=4) at 2.5, 7.5, and 75 ng/mL were also extracted and analyzed. Table 2 summarizes R2 values from the calibration curves and QC summary data for all compounds. Quality control (QC) results were accurate and precise at low, medium and high concentrations. Accuracies for low level QC samples (2.5 ng/mL) ranged from 90-111% with an average of 101%. The results for the medium and high QC levels were excellent for all analytes, with all accuracies within 15% of expected values. Analytical precision was excellent with most % RSDs less than 10% and none greater than 15%. When accuracy was assessed over all levels (low, medium, and high), the means ranged from 92% to 107%. Limits of detection were as low as 0.1 ng/mL for some of the analytes and none were greater than 2 ng/mL. This was sufficient for the performance requirements of the assay, as these compounds are typically measured in the ng/mL range.

A panel of 22 synthetic cannabinoid drugs and metabolites were extracted from urine and analyzed by UPLC-MS/MS. The use of Oasis HLB μElution plates enabled the simultaneous extraction of acidic, basic and neutral compounds, ensuring that a wide variety of compounds and metabolites could be analyzed. Separation using the CORTECS UPLC C18 Column enabled the analysis of all compounds in a short analysis time with baseline resolution of critical isobaric pairs. Separation efficiency and peak widths were superior to fully porous columns of matching dimensions and particle size. This method enables the rapid and reliable extraction and analysis of this critical class of compounds for forensic toxicology. The excellent performance seen on this variety of compounds and the universal nature of the extraction method should allow its use on other synthetic cannabinoids and metabolites, an important feature given the rapid development of new, related compounds.
720004780, September 2013