
For research use only. Not for use in diagnostic procedures.
This study presents the results of screening using a method based on UPLC in combination with Tof-MSE. The use of UPLC-Tof-MSE offers unique benefits for drug screening applications due to the acquisition of an unrestricted dataset with excellent sensitivity. Accurate mass measurement allows the prediction of elemental composition, which is particluarly beneficial when dealing with situations involving potenetial emerging drugs and analogs.
Recreational drug use is common in the UK, particularly among those frequenting nightclubs and others within the night-time entertainment community. The British Crime Survey of 2010/2011 estimated that 8.8% of the adult population had used illicit drugs in the last year;1 whereas, an on-line survey, conducted over the same period by the dance magazine Mixmag, showed significantly higher use in the population who frequent the night-time establishments. The survey reported that 50% to 75% of attendees had used MDMA (ecstasy), cocaine, or mephedrone over the previous year.2 Furthermore, a more recent survey of attendees at a London nightclub indicated that 41% of those surveyed claimed to have used mephedrone over the last month.
In a study designed to assess the feasibility of using pooled urine to confirm which drugs are currently being used, a series of samples were collected using an adapted portable urinal at a London nightclub.3
Samples were analyzed using a variety of analytical techniques. This study presents the results of screening using a method based on UPLC in combination with Tof-MSE. The use of UPLC-Tof-MSE offers unique benefits for drug screening applications due to the acquisition of an unrestricted dataset with excellent sensitivity. Accurate mass measurement allows the prediction of elemental composition, which is particularly beneficial when dealing with situations involving potential emerging drugs and analogs.
A series of four pooled urine samples were collected during two separate events using a modified portable urinal placed on-site at a large south London nightclub.
|
Event 1: |
Friday/Saturday (11:00 pm to 4:00 am) |
|
Collection times: |
2:00 am (sample #1), 3:00 am (sample #2), and 4:00 am (sample #3) |
|
Event 2: |
Saturday/Sunday (11:00 pm to 10:00 am) |
|
Collection time: |
10:00 am (sample #4) |
|
Use was both voluntary and anonymous. |
Urine samples were transferred to a vial and diluted 5-fold with mobile phase.
|
System: |
ACQUITY UPLC |
|
Column: |
ACQUITY UPLC HSS C18 2.1 x 150 mm, 1.8 μm, part number 186003534 |
|
Column temp.: |
50 °C |
|
Sample temp.: |
10 °C |
|
Injection volume: |
10 μL |
|
Mobile phase A: |
5 mM ammonium formate, pH 3 |
|
Mobile phase B: |
Acetonitrile containing 0.1% formic acid |
|
Weak wash: |
Mobile phase A |
|
Strong wash: |
Mobile phase B |
|
Gradient: |
15-min gradient flow rate at 400 μL/min |
|
Mass spectrometer: |
Xevo G2 QTof |
|
Ionization mode: |
ESI+ |
|
Capillary voltage: |
0.8 kV |
|
Cone voltage: |
20 V |
|
Acquisition mode: |
MSE |
|
Collision energy: |
Ramped from 10 to 40 eV (MSE) |
MassLynx robust software solutions including ChromaLynx, TargetLynx, and Posi±ive Application Manager in targeted analysis mode
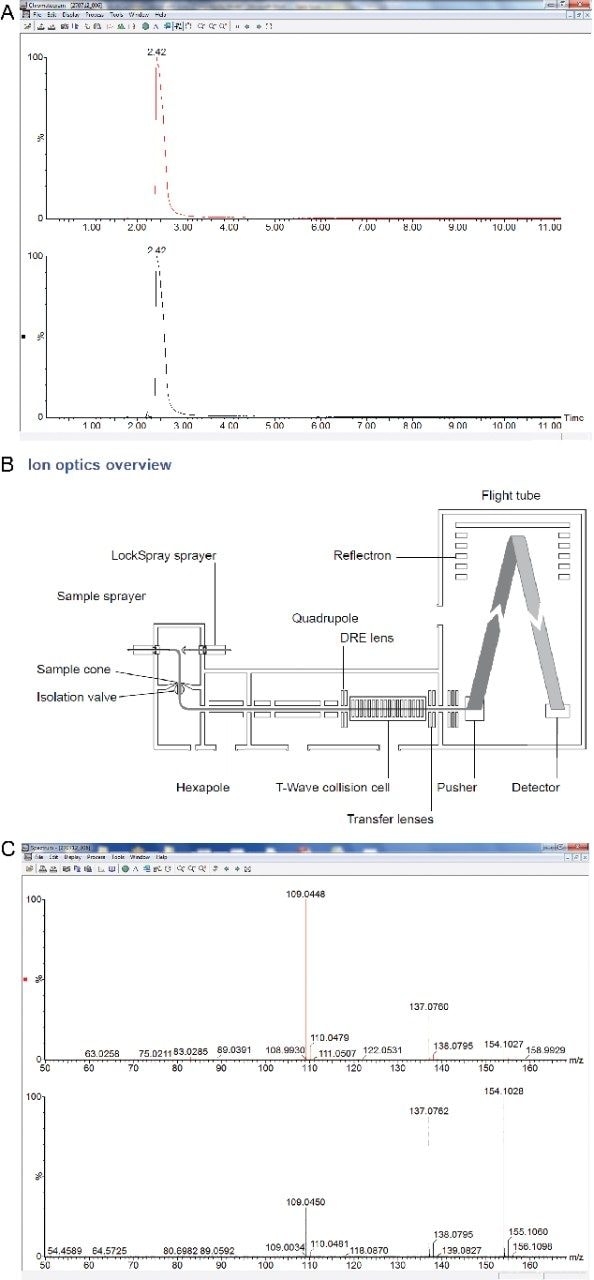
Data was collected using a Waters Xevo G2 QTof in MSE mode, involving the rapid alternation between two energy conditions thus, providing the accurate mass of the precursor ion, in addition to fragment ions, for further confirmatory purposes, as shown in Figure 1. Acquired data were then compared to a comprehensive database, prepared under the same conditions, containing 1000 drugs and metabolites. All substances contained within the database have an associated retention time (RT) with >75% of entries containing additional confirmatory ion data. Substance identification is, thus, based on retention time and a mass ‘fingerprint’ for each analyte, the latter comprising accurate mass of the precursor ion and up to four fragment ions.
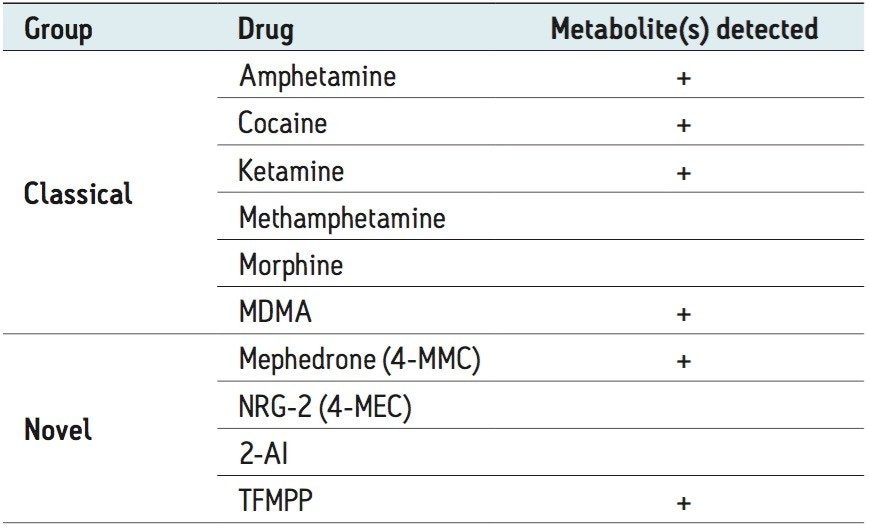
A total of 72 parent drugs and their metabolites were detected in the four samples. Detected drugs could be broadly divided into the following categories:
Each of the four samples contained several of the substances listed in Table 1. Detection of the metabolites confirmed the presence of drugs that were actually being used and metabolized by individuals rather than measuring unused drug materials that had simply been discarded into the urinal.
A number of potential adulterants were also detected in the samples including the following: diltiazem, levamisole, caffeine, lidocaine, and quinine. In addition, prescription or over-the-counter medications identified included anti-depressants, benzodiazepines and other sedatives, anti-histamines, anti malarials, anti-virals, nasal decongestants, analgesics, and proton pump inhibitors.
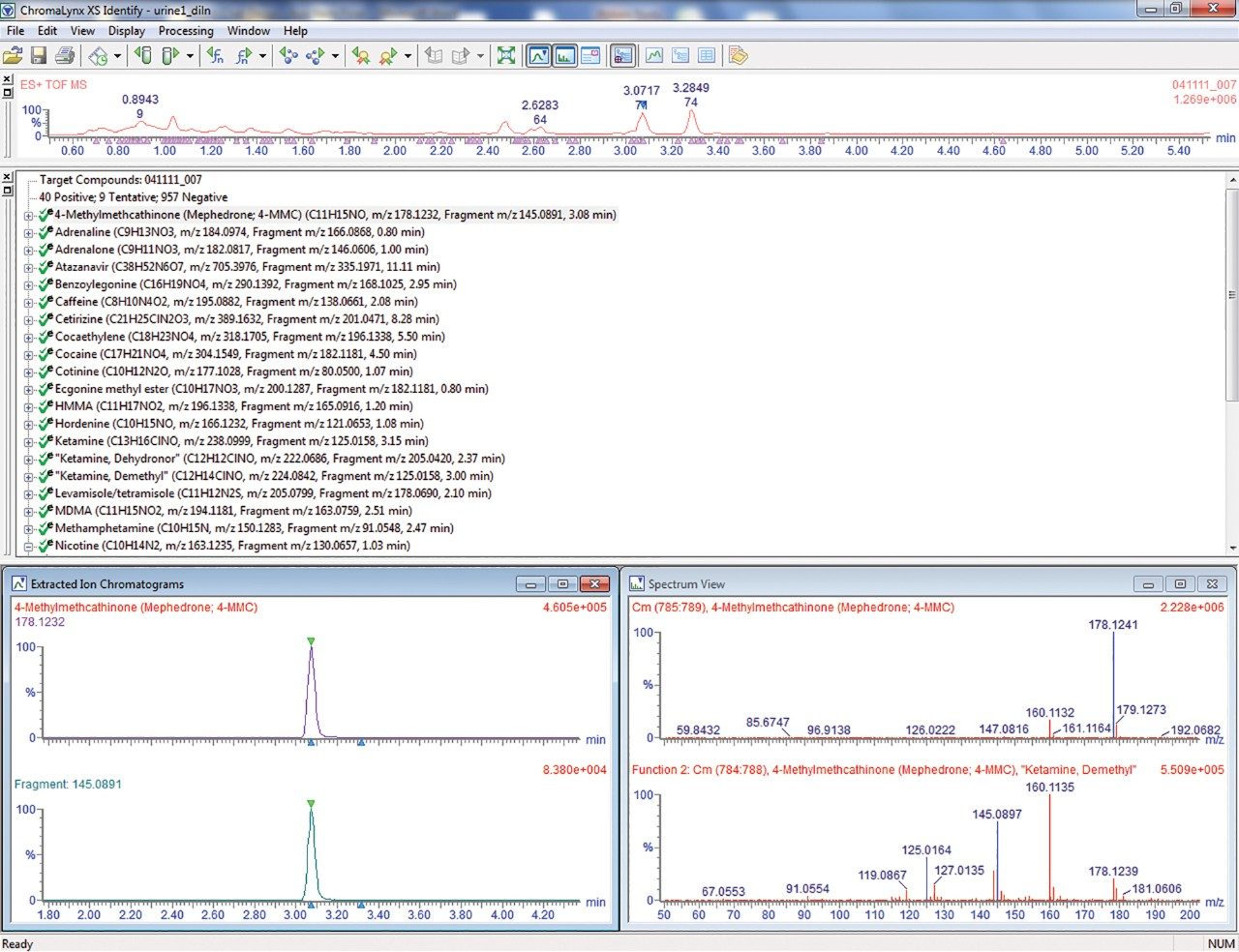
Broad screening techniques used to identify drugs of interest from urine samples provide a critical tool in the effort to understand regional recreational drug usage patterns. Use of a pooled sample provides a viable, anonymous biological specimen while avoiding issues related to the limitations and variability associated with self-reporting methods. This method, performed on a Xevo G2 QTof, demonstrates a sensitive, robust method that can analyze diluted urine samples and screen against an extensive database containing 1000 toxicologically relevant compounds. Ultimately, this method establishes a foundational technique that can be utilized to establish trends in recreational drug use across any time, geographic, or demographic profile.
A full validation by the user would be necessary prior to adoption in a laboratory.
720004583, June 2013