
To demonstrate that the combination of the DisQuE Sample Preparation Kit with UPLC-FLR provides a rapid screening tool for the detection of PAHs in seafood.
Major oil spills, such as the Exxon Valdez in 1989 and the April 2010 Gulf of Mexico oil spill, have raised concerns over the quality of seafood harvested from these regions. Fish, crustaceans, and mollusks may come into contact with, or ingest the oil thereby introducing potential health risks to consumers.
Of the many compounds found in oil, an important subset is the Polyaromatic Hydrocarbons (PAHs). The US Environmental Protection Agency (US EPA) has defined these compounds as priority pollutants.1 The US Food and Drug Administration (US FDA) has also established levels of concern ranging from 3.5 x 10-2 mg/kg benzo(a) pyrene in finfish, to 2.0 x 103 mg/kg combined phenanthene and anthracene in oysters.2 Confirmatory analysis is required if any PAHs are detected at half the level of concern.2
To prevent consumption of contaminated seafood and minimize the impact on the seafood industry, a fast screening method is required to analyze these compounds of concern at the stated levels. Here we demonstrate that, following a simple extraction method using Waters DisQuE Dispersive Sample Preparation Kit (QuEChERS), an analysis of PAHs can be achieved in less than 4 minutes using the ACQUITY UPLC H-Class System with Fluorescence Detection.

|
System: |
ACQUITY UPLC H-Class with Large Volume Flow Cell (LVFC) |
|
Column: |
PAH 4.6 x 50 mm, 3 μm |
|
Column temp.: |
35 °C |
|
Injection volume: |
10 μL |
|
Sampling rate: |
20 pts/sec |
|
Detection: |
Fluorescence using timed programmed wavelength changes |
|
Software: |
Empower 2 |
|
Mobile phase A: |
Milli-Q water |
|
Mobile phase B: |
Methanol, Fisher Optima Grade |
|
Mobile phase C: |
Acetonitrile, Fisher Optima Grade |
|
Standards: |
PAH Certified Standard, AccuStandard M 8310 |
|
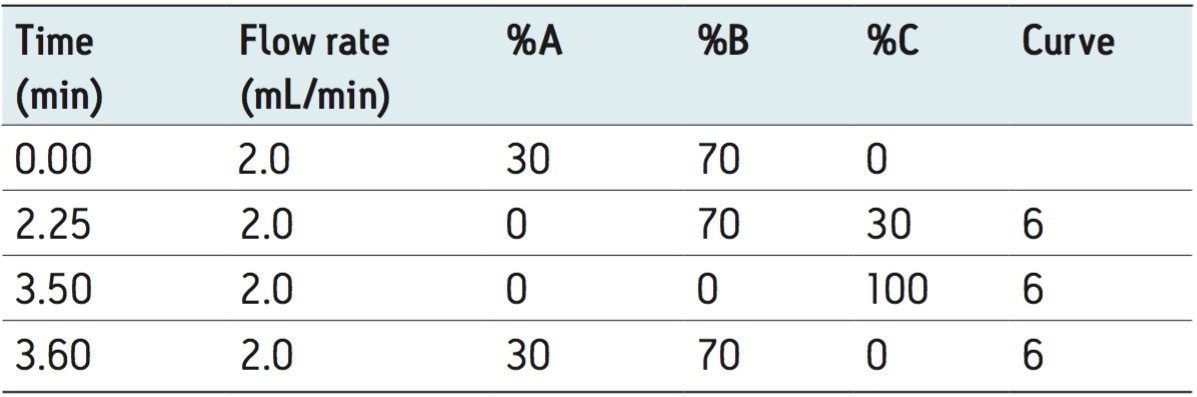
Flow rate: |
2.0 mL/min |
Individual samples of fish fillets (flounder), shelled shrimp, and shucked oysters with liquor were homogenized using a food processor per the method described by Ramalhosa et. al.3 15 grams of each homogenized tissue were added to individual centrifuge tubes and spiked at three different levels, 50 ng/g, 1 μg/g, and 10 μg/g for shrimp and oysters, 15 ng/g, 1 μg/g, and 10 μg/g for fish, with a spiking solution prepared from the certified PAH standard. 5 mL of water were added to the fish and shrimp samples to aid mixing. The oysters did not need extra liquid. The spiked samples were thoroughly mixed and allowed to sit at room temperature for an hour.
To each centrifuge tube was added the contents of a DisQuE tube (P/N 186004571), 6 g magnesium sulfate + 1.5 g sodium acetate, and 15 mL of acetonitrile. The centrifuge tube was shaken vigorously for at least one minute to produce an emulsion of seafood tissue, buffer salts and acetonitrile. Here also the procedure of Ramalhosa3 was followed as no acetic acid was added to the acetonitrile, nor was a secondary PSA cleanup step carried out. Initial work in our laboratory confirmed that the PSA step was not required for LC-FLR analysis (data not shown). After centrifuging at 3000 rpm for 5 minutes, a portion of the clear acetonitrile supernatant layer was transferred to an autosampler tube for direct injection. The 1 μg/g and 10 μg/g spikes were diluted with acetonitrile 1:10 and 1:100 respectively. Samples were quantified using a six-point linear calibration curve. Standards were prepared by diluting the certified standard with acetonitrile.
Dispersive sample preparation, often referred to as QuEChERS, is a well proven and fast sample preparation method for the analysis of pesticides in food commodities.4 More recently, this method has been used to extract other contaminants from food matrices, including polyaromatic hydrocarbons.3
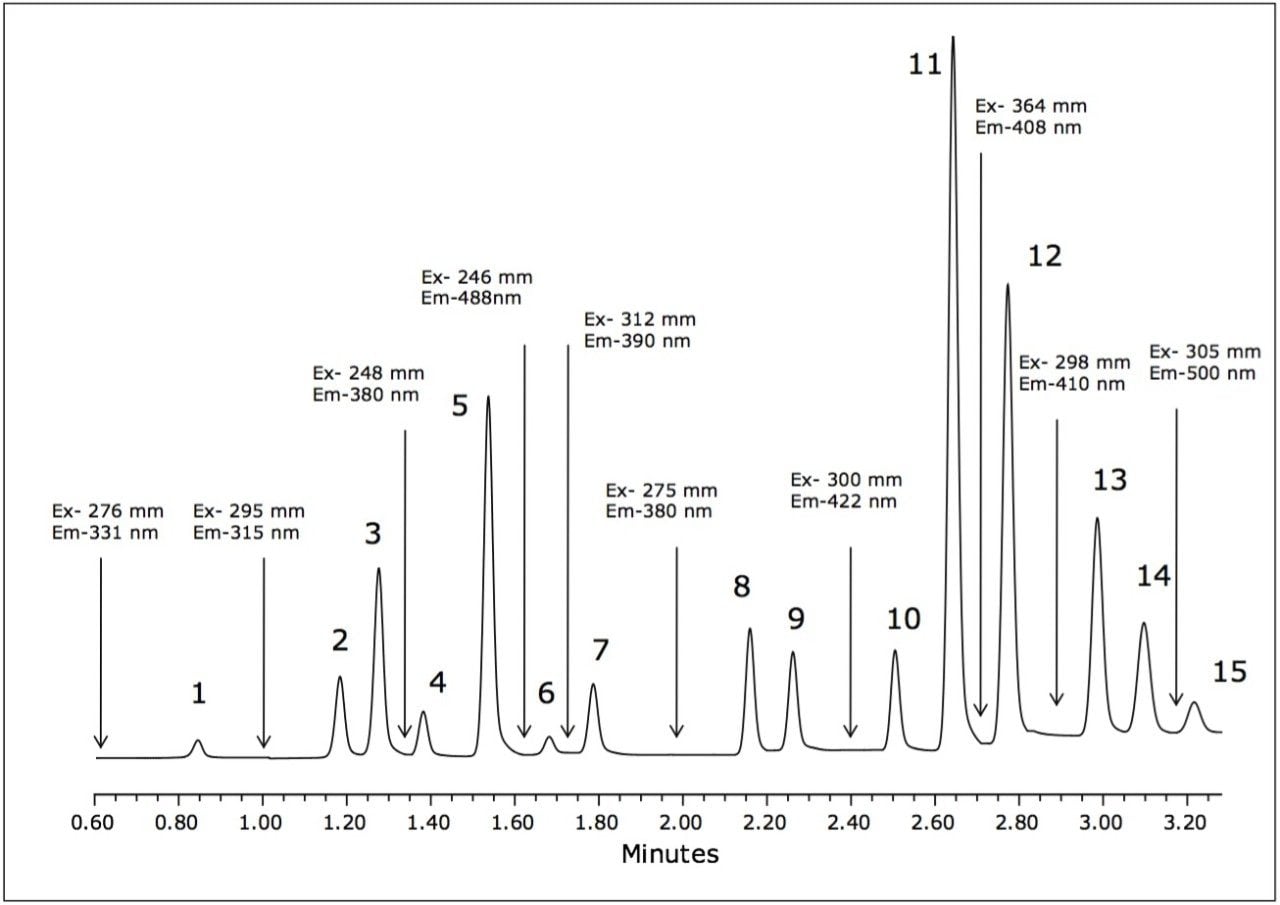
The separation of the 15 fluorescent PAHs that are listed as priority pollutants by the US EPA was achieved in only 3.5 minutes using the ACQUITY UPLC H-Class System. The separation of the analytes is shown in Figure 2, with the timed programmed wavelength changes indicated by arrows.
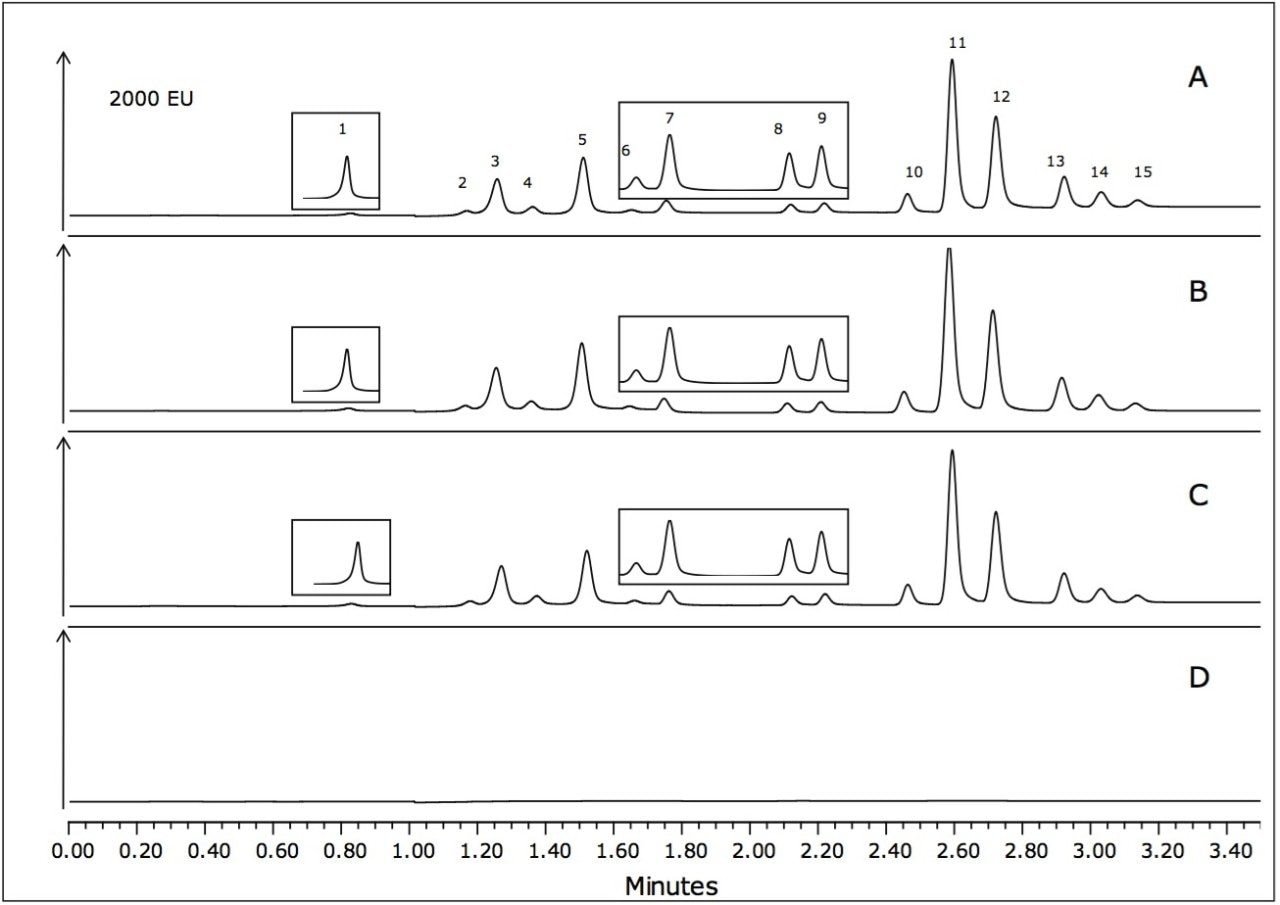
Example chromatograms of the shrimp, fish, and oyster matrices spiked at 10 μg/g are shown in Figure 3. Certain sections of the chromatograms have been magnified to more clearly show the peaks of interest. As shown in Figure 3D, the blank water sample that was also carried through the sample preparation procedure shows a very clean chromatogram.
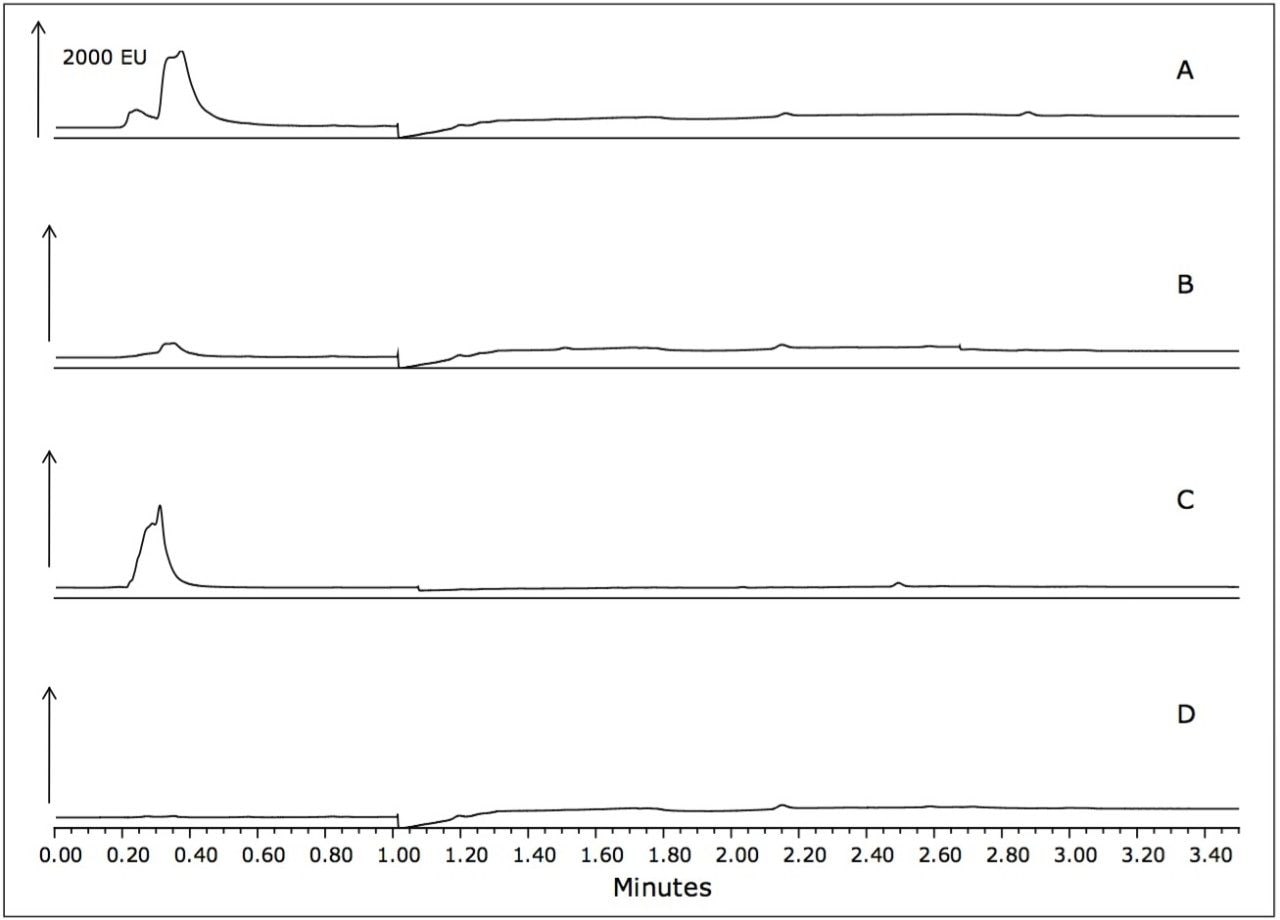
Samples of unspiked seafood matrices that were used in this sample preparation procedure also showed no matrix interference, as shown in Figure 4.
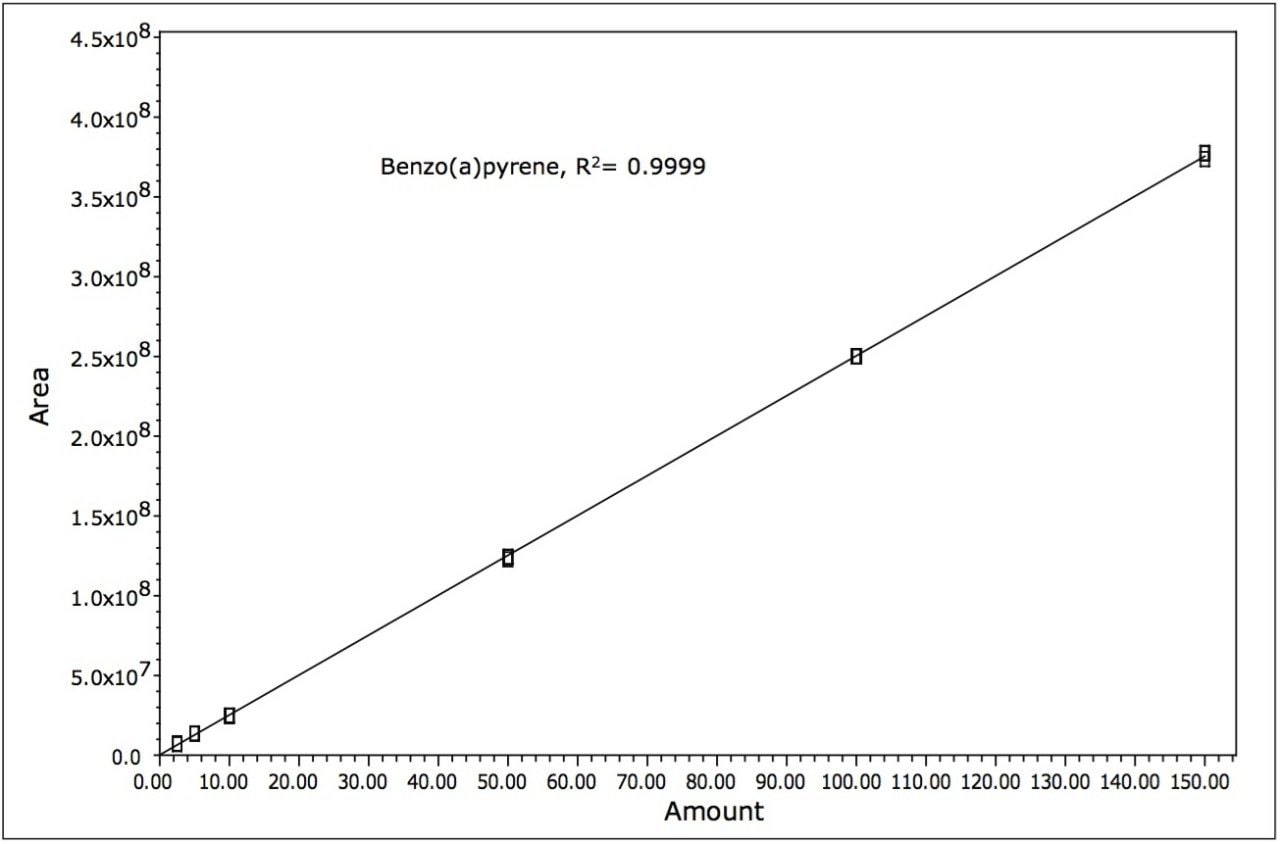
Samples were quantified against six point calibration curves of each of the analytes. An example calibration curve is shown for benzo(a)pyrene in Figure 5. Linearity (R2) was > 0.995 for all analytes.
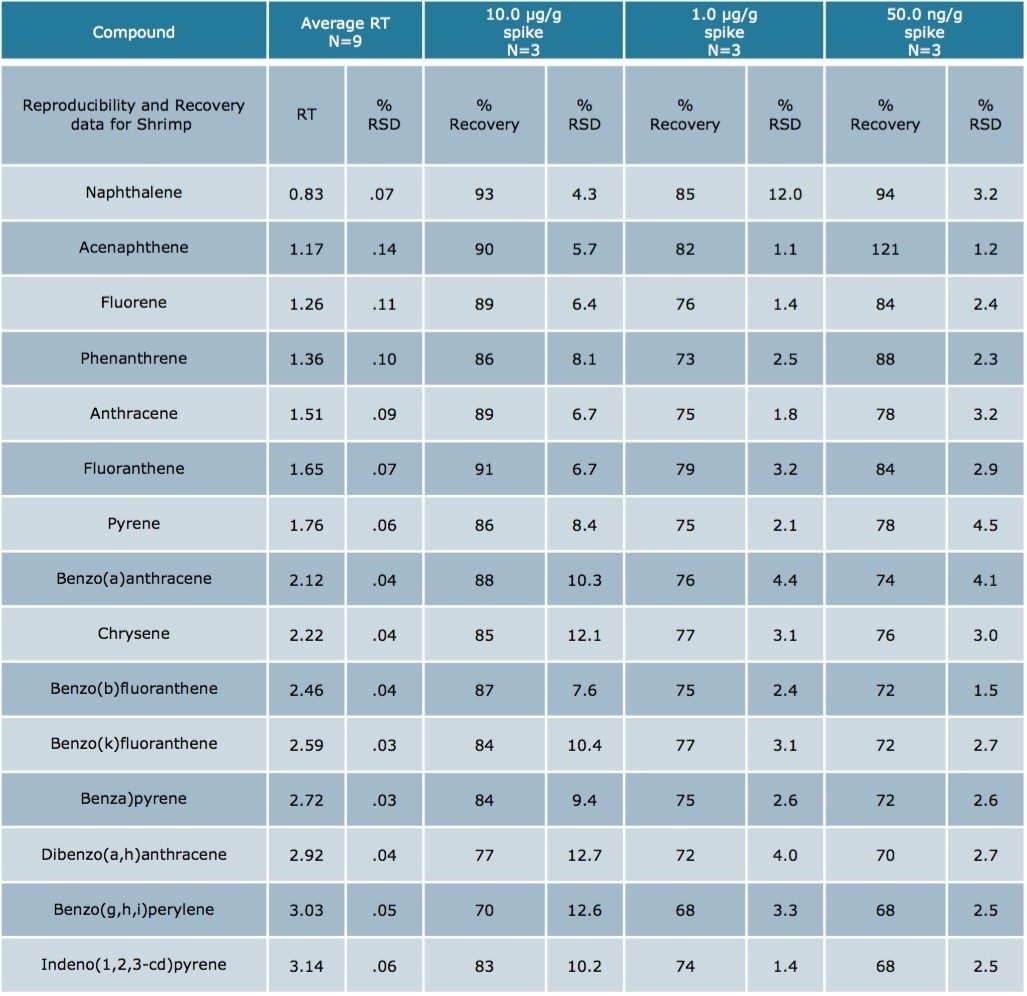
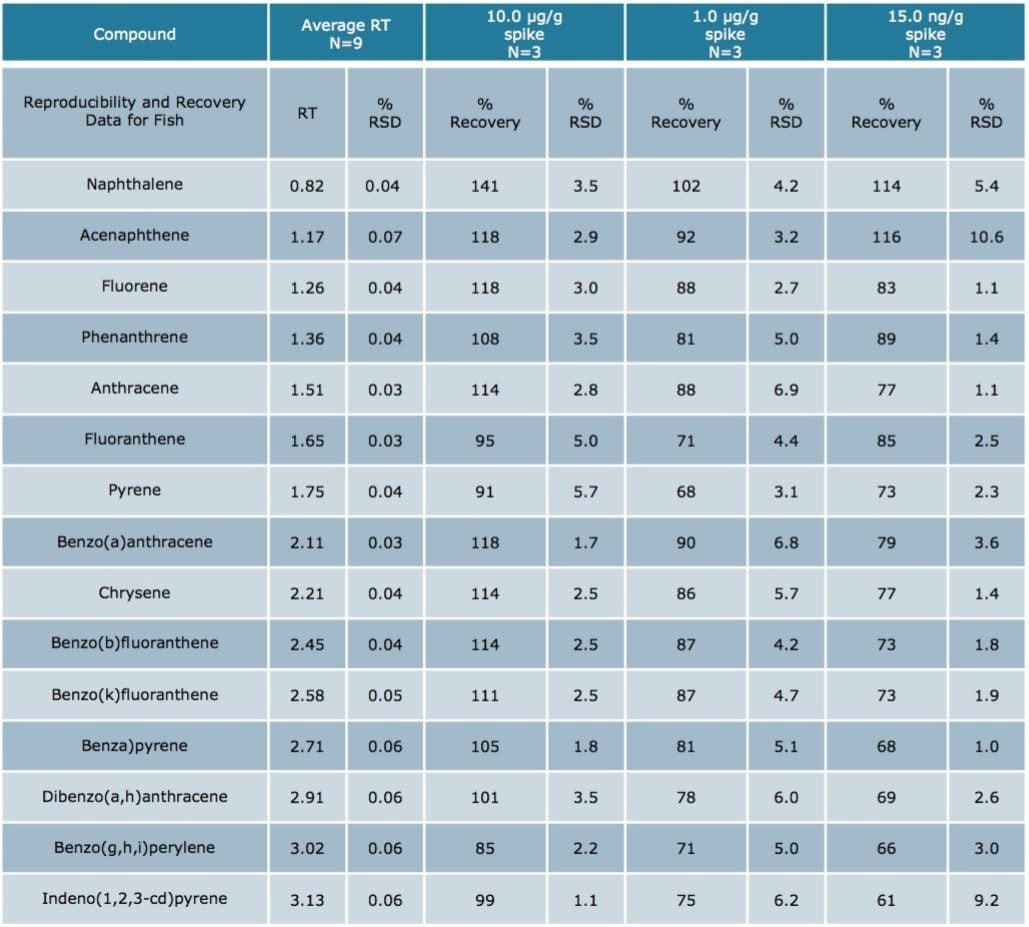
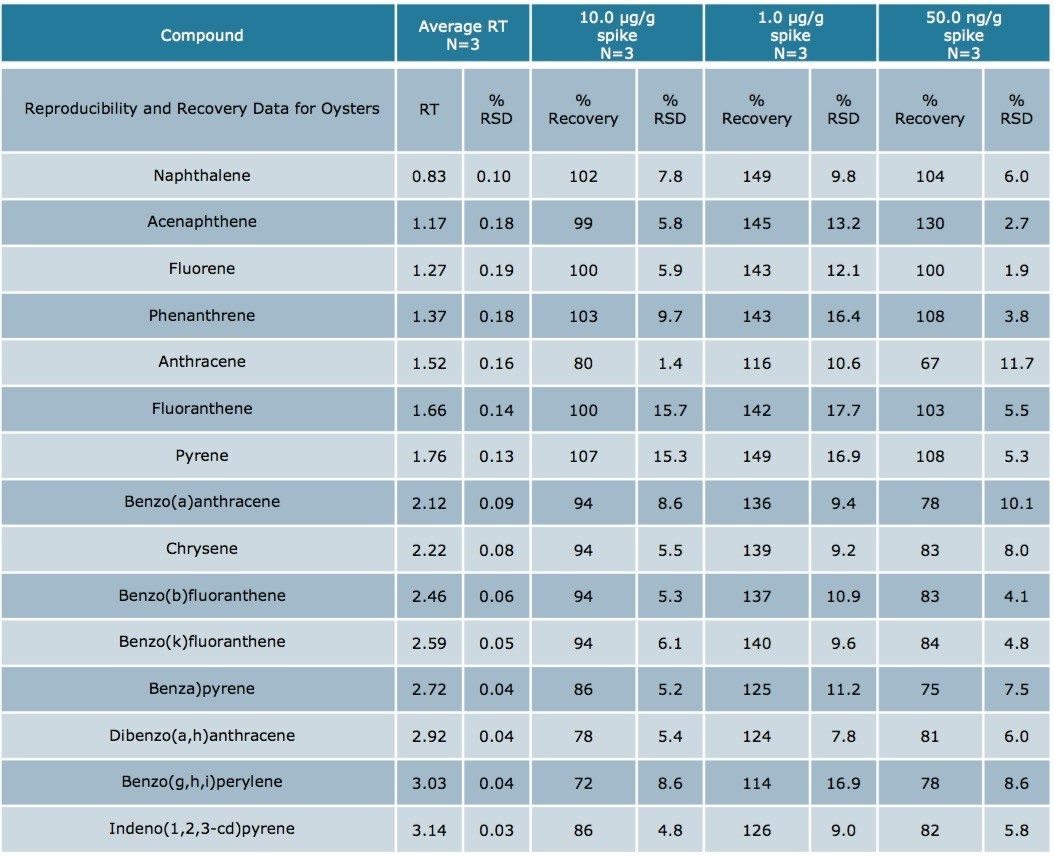
Using Waters DisQuE Dispersive Sample Preparation Kit, PAHs were extracted from three different seafood matrices. The recoveries and percentage RSDs for shrimp, fish, and oysters are shown in Tables 1 to 3. Recoveries were in the range of 68% to 149%. Table 4 lists the recoveries for a series of QC water spikes, fortified at the levels listed and carried through the sample prep procedure previously described.
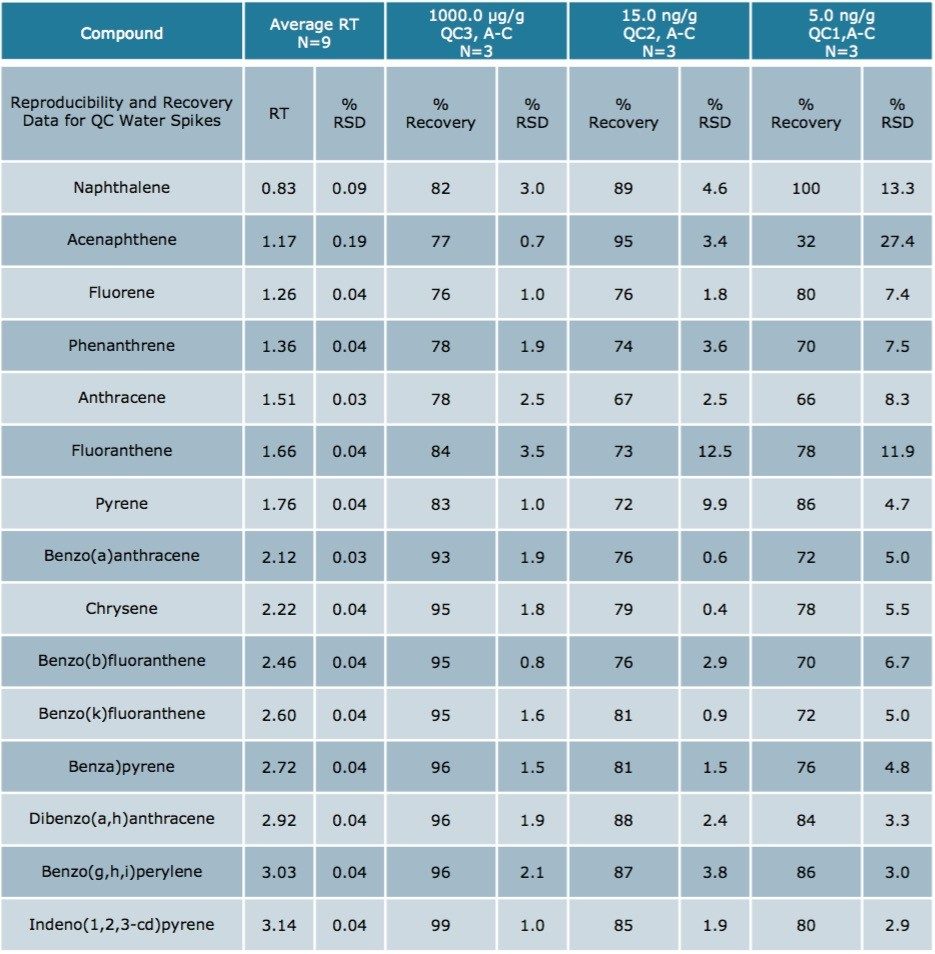
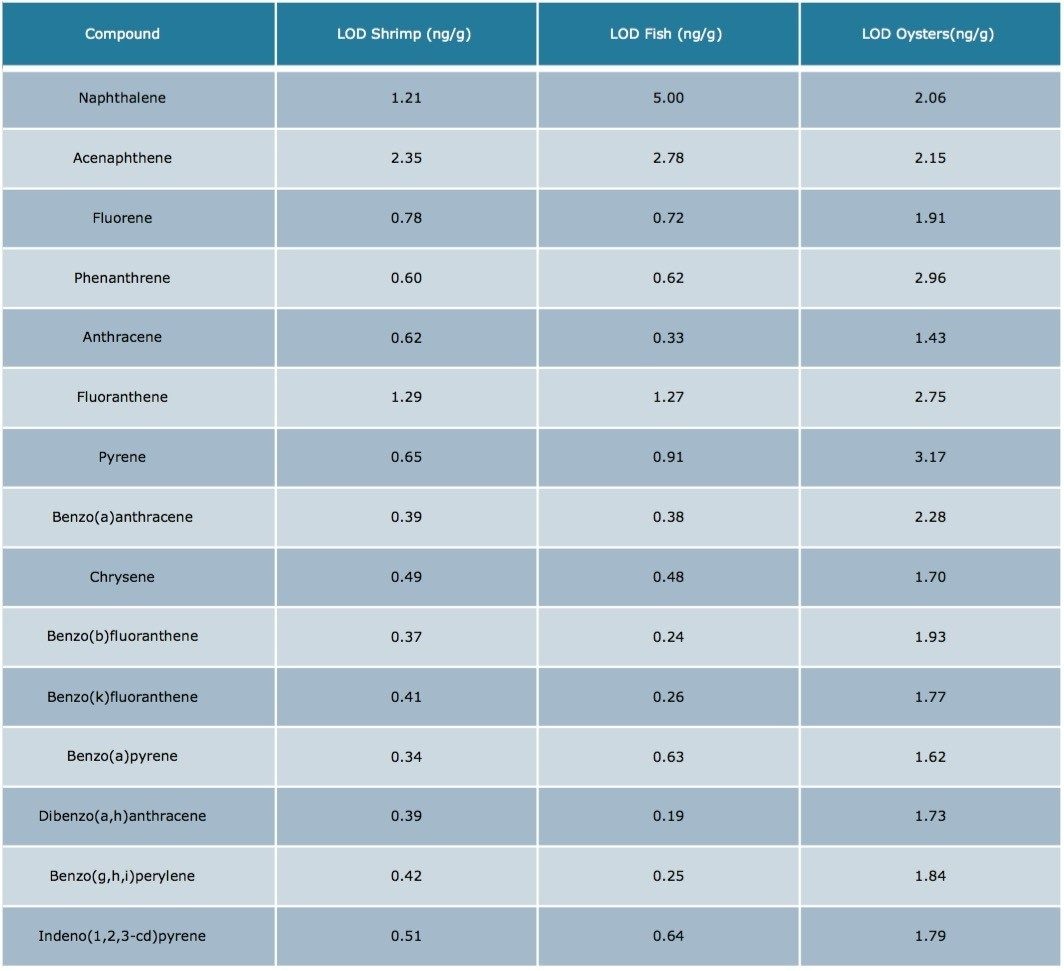
The results were excellent for all of the compounds at each fortification level, except the lowest level for acenapthene in water (5 ng/g). At this low level, acenapthene showed more variation owing to the small peak area and a sloping baseline that was only noticeable at this level. Table 5 is an estimation of the Limit of Detection based on seven replicates of each seafood matrix spiked at a 5 ng/g level, and calculated per US EPA 40 CFR, Appendix B to part 136 Rev 1.15.
This application note demonstrates that the combination of the DisQuE Sample Preparation Kit with LC-FLR provides a rapid screening tool for the detection of PAHs in seafood.
720003891, December 2013