
For forensic toxicology use only.
A strategy for the successful extraction and analysis of representatives of several different classes of synthetic cathinones from human urine samples for forensic toxicology has been described here. Using mixed-mode solid phase extraction (SPE) followed by UPLC-MS/MS analysis, a panel of 10 synthetic cathinones was extracted with excellent recovery and analytical sensitivity.
Synthetic cathinones, commonly marketed as “bath salts,” are variations of the chemical cathinone, naturally found in the Khat plant (Catha edulis). These drugs are central nervous system stimulants, mimicking the effects of drugs such as amphetamine, methamphetamine, cocaine, and methylphenidate. Often labeled as “not for human consumption,” their popularity and use have increased substantially in the last several years1. In addition, new drugs with modifications to existing cathinone structures are constantly being developed and marketed in order to circumvent drug of abuse legislation aimed at specific compounds. This current application note details a strategy for the successful extraction and analysis of representatives of several different classes of synthetic cathinones from human urine samples for forensic toxicology. Using mixed-mode solid phase extraction (SPE) followed by UPLC-MS/MS analysis, a panel of 10 synthetic cathinones was extracted with excellent recovery and analytical sensitivity. Matrix effects were minimal for all compounds, and calibration curves were linear from one to 500 ng/mL. The analysis of several different classes of these drugs should render this method applicable to newly developed related compounds with minimal, if any, modification necessary.
|
LC conditions |
|
|---|---|
|
System: |
ACQUITY UPLC |
|
Column: |
ACQUITY UPLC BEH C18 1.7 μm, 2.1 x 100 mm (p/n 186002352) |
|
Column temp.: |
30 °C |
|
Injection volume: |
10 μL |
|
Flow rate: |
0.5 mL/min |
|
Mobile phase A: |
0.1% formic acid in MilliQ water |
|
Mobile phase B: |
0.1% formic acid in ACN |
|
Gradient: |
Initial hold at 20% B for 0.5 minute, increased to 30% over 2 minutes, then returned to 20% over 0.1 minute. The system was allowed to re-equilibrate for 1.4 minutes. The entire cycle time was 4.0 minutes. |
|
Vials/plates: |
96-well sample collection plates, 700 μL (p/n 186005837) |
|
Mass spectrometer: |
Xevo TQD |
|
Ionization mode: |
ESI positive |
|
Acquisition mode: |
MRM (See Table 1 for transitions) |
|
Capillary voltage: |
1 kV |
|
Collision energy (eV): |
Optimized for individual compounds (See Table 1) |
|
Cone voltage (V): |
Optimized for individual compounds (See Table 1) |
All data was acquired and analyzed using Waters MassLynx Software
4-methylmethcathinone (Mephedrone, 4-MMC), 3,4-methylenedioxy- N-methylcathinone (methylone), and methylenedioxypyrovalerone (MDPV) were purchased from Cerilliant (Round Rock, TX). All other compounds and metabolites were purchased from Cayman Chemical (Ann Arbor, MI).
A combined stock solution of all compounds (5 μg/mL) was prepared in methanol. Working solutions were prepared daily by preparing standards in matrix (urine), and performing serial dilutions to achieve the desired concentrations. Calibrator concentrations ranged from five to 500 ng/mL for all analytes.
The 10 compounds analyzed, listed in Table 1, constitute a panel which includes forensically relevant cathinones such as pyrrolidiniophenones (α-PPP, α-PVP), methylenedioxycathinones (methylone, ethylone), and methoxymethcathinones (methedrone). All are weak bases of moderate hydrophobicity that are well suited to extraction by mixed-mode ion exchange. Cone voltages, MRM transitions, and respective collision energies are listed for all compounds in Table 1.
Samples were extracted using mixed-mode, strong cation SPE. For each sample, 100 μL of urine was pre-treated by adding an equal volume of 4% H3PO4. Wells of the 96-well Oasis MCX μElution Plate (p/n 186001830BA) were conditioned with 200 μL MeOH, followed by 200 μL MilliQ water. 200 μL of each diluted sample was then added to each well, resulting in a sample load of 100 μL urine. After loading, the wells were washed with 200 μL of aqueous 2% formic acid, followed by 200 μL MeOH. All samples were then eluted with 2 x 50 μL of 60:40 ACN/IPA containing 5% by volume of a concentrated NH4OH solution (Fisher, 20% to 22%). Samples were then neutralized with 5 μL of concentrated formic acid, and diluted with 100 μL of water. 10 μL was injected onto the LC-MS/MS system.
Matrix effects were calculated according to the following equation:

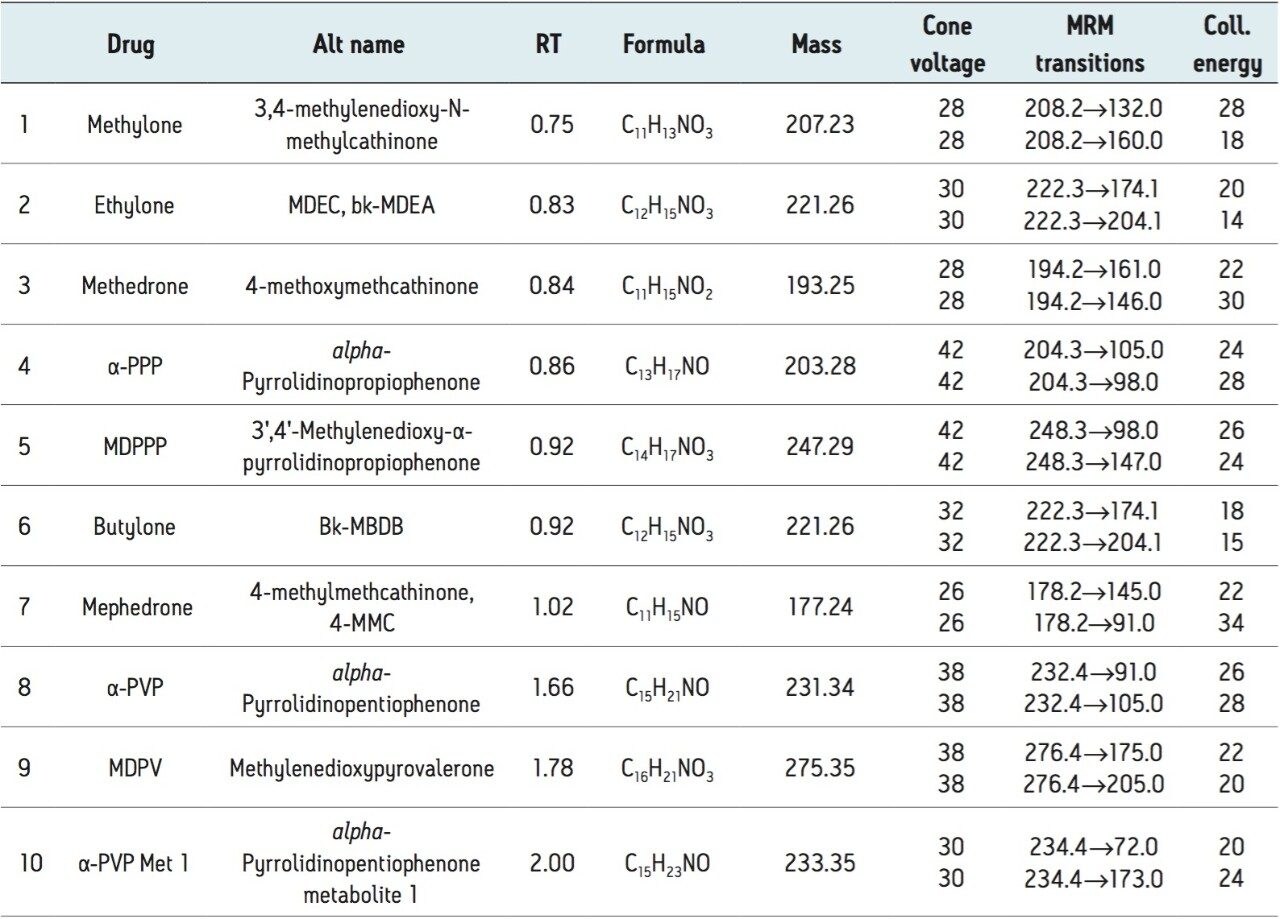
A representative chromatogram of all compounds from a 50-ng/mL calibration standard is shown in Figure 1. Peak assignments are listed in Table 1. Using an ACQUITY UPLC BEH C18 1.7 μm, 2.1 x 100 mm Column, all analytes were analyzed within two minutes with a total cycle time of four minutes. Ethylone and butylone (peaks 2 and 6), an isobaric pair of compounds with identical precursor and product ions, demonstrate baseline resolution despite the short analysis time, enabling unambiguous identification that would not be possible if the two compounds co-eluted. Peak shape was excellent for all compounds, with no significant tailing or asymmetries, and all peak widths were under four seconds.
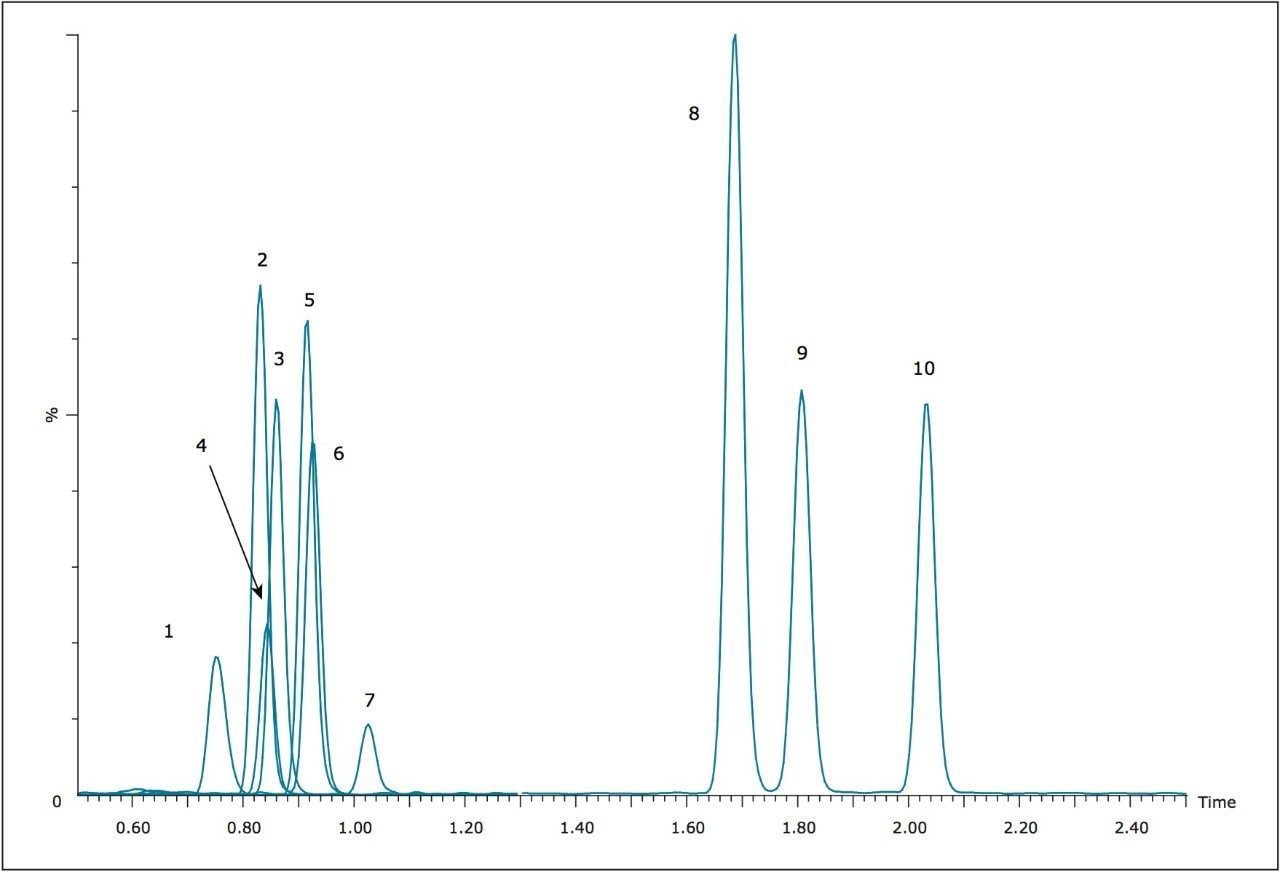
For this application, elution from Oasis MCX μElution plates was initially performed with a solution of 60:40 ACN/MeOH containing 5% concentrated NH4OH. Recoveries were good for most compounds, averaging approximately 80%. However, the α-PVP hydroxyl metabolite was recovered at only 40%. In addition, significant matrix effects, mostly in the form of ion suppression, were seen for many of the compounds tested, especially the three earliest eluting compounds, methylone, ethylone, and methedrone. Replacing the MeOH in the elution solvent with water did not reduce the matrix effects seen, and reduced the recovery of the α-PVP hydroxyl metabolite to only 4%. However, when the MeOH was replaced with isopropanol (IPA), the recovery of the α-PVP hydroxyl metabolite was increased to 87.4%, and the recoveries for the other nine analytes were improved from an average of 81% to nearly 98%. In addition, matrix effects were nearly eliminated for all compounds. Figure 2 summarizes the recoveries and matrix effects seen with the final extraction method. Recoveries range from 87.4% to 100.5% with matrix effects ranging from -3.6% to 12.4% at an average of 6.2%. This extraction protocol results in almost complete recovery, and minimizes matrix effects for the compounds tested.
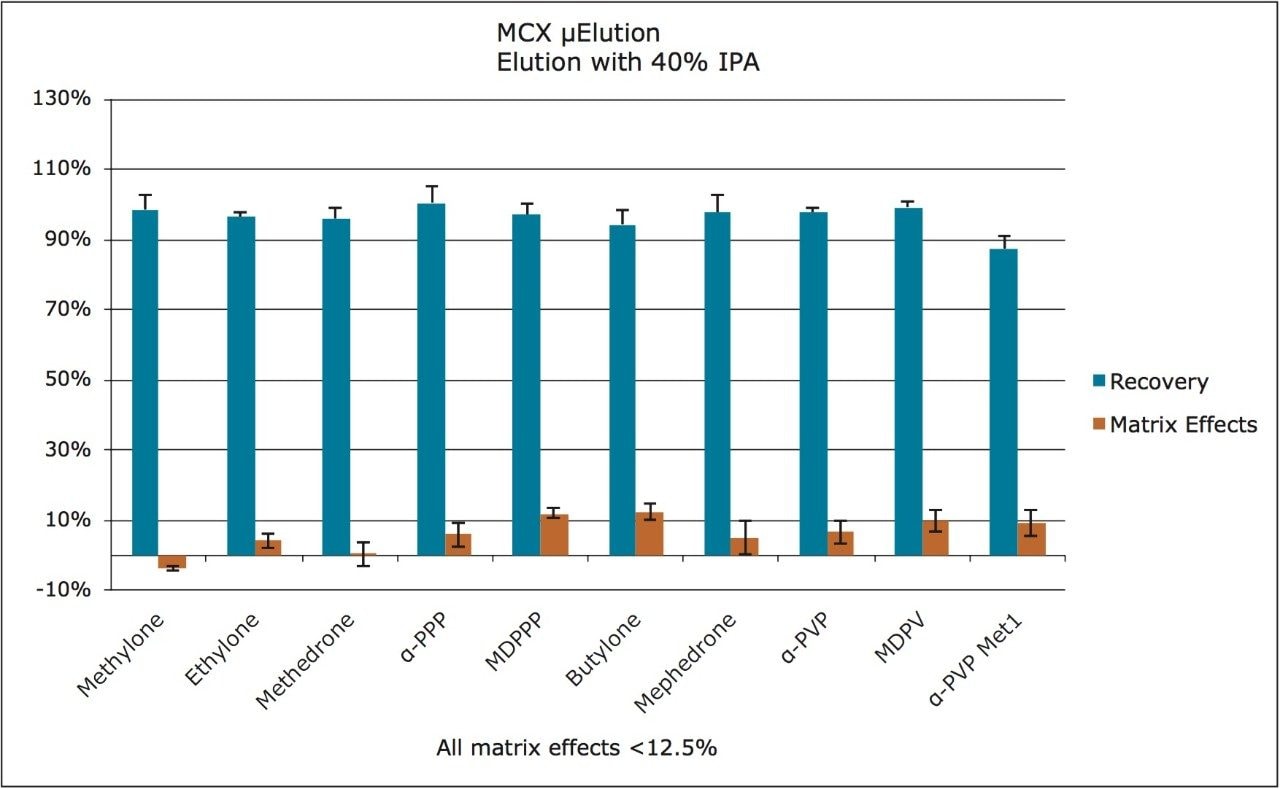
In order to assess linearity and analytical sensitivity, calibration curves were extracted at concentrations ranging from 1 to 500 ng/mL for all components. Table 2 summarizes R2 values and average deviations (N=3) from nominal values for all compounds. With the exception of the 10 ng/mL point for the α-PVP metabolite, nearly all calibration points were within 15% of their target values. At the 1 ng/mL level, peak areas for all compounds were at least five-fold higher than that of blank, extracted urine samples, and all were within 5% of the nominal value.
A panel of 10 synthetic cathinone drugs were extracted from urine by mixed-mode SPE, and analyzed by UPLC-MS/MS. The use of Waters MCX μElution plates resulted in excellent recoveries for all analytes while minimizing matrix effects. Furthermore, no evaporation or reconstitution steps were necessary, saving time and eliminating the risk of sample loss by evaporation or adsorption that can accompany such procedures. Separation by UPLC enabled the analysis of all compounds in two minutes with baseline resolution of a critical isobaric pair. Calibration curves were linear from one to 500 ng/mL with limits of quantitation of 1 ng/mL for all compounds. This method enables the rapid and reliable extraction and analysis of this critical class of compounds for forensic toxicology.
720004708, June 2013