In this application note, Ostro 96-well sample preparation plates are used to eliminate both proteins and the vast majority of PLs while maintaining high analyte recovery, all with a simple single step method. A screening method for a group of 26 structural analogs and metabolites in plasma was developed using this technique.
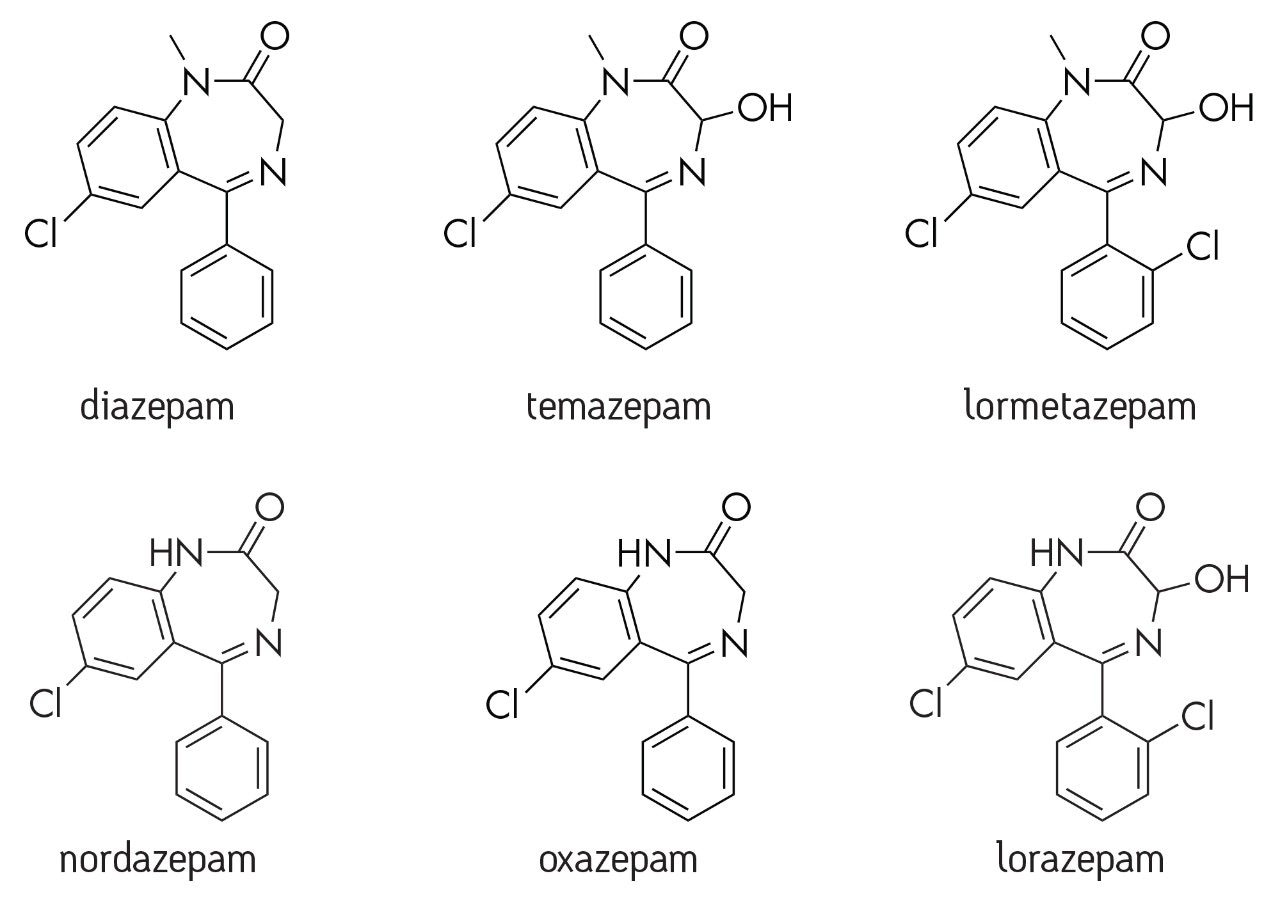
Drug discovery is a vital segment of pharmaceutical research where vast numbers of compounds are screened to determine therapeutic efficacy, activity, and ADME properties. This process helps identify the handful of drug candidates that will progress further. Many closely related drug compounds must be rapidly analyzed and quick decisions must be made as to which drugs will continue into development and eventually clinical trials. During the drug discovery stage, speed, time, ease of use, and high throughput are key aspects of everyday work. There is little time for method development, making simple and universal sample prep methods such as protein precipitation (PPT) an attractive choice. Crude techniques such as PPT are often quite efficient in terms of generating high analyte recovery but result in relatively dirty samples. In particular, PPT does little to eliminate phospholipids (PLs), a major source of concern in bioanalysis. PLs build up in LC-MS/MS systems and are one of the major sources of matrix effects in plasma-based assays. Amongst other problems, matrix effects also alter mass spectrometry response in an unpredictable manner, decrease method robustness, and add to method variability. In this publication, Ostro 96-well sample preparation plates are used to eliminate both proteins and the vast majority of PLs while maintaining high analyte recovery, all with a simple single step method. A screening method for a group of 26 structural analogs and metabolites (see Figure 1 for representative structures) in plasma was developed using this technique.
|
Column: |
ACQUITY UPLC BEH C18, 2.1 x 50 mm, 1.7 μm |
|
Mobile Phase A: |
0.1% HCOOH in H2O |
|
Mobile Phase B: |
Methanol |
|
Flow Rate: |
0.6 mL/min |
|
Injection Volume: |
18.0 μL |
|
Injection Mode: |
Partial Loop |
|
Column Temperature: |
35 °C |
|
Sample Temperature: |
15 °C |
|
Strong Needle Wash: |
60:40 ACN:IPA + 0.2% conc. HCOOH (600 μL) |
|
Weak Needle Wash: |
80/20 H2O/MeOH (200 μL) |
|
Time (min) |
Profile |
Curve |
|
|---|---|---|---|
|
%A |
%B |
||
|
0.0 |
98 |
2 |
6 |
|
2.0 |
1 |
99 |
6 |
|
2.5 |
1 |
99 |
6 |
|
2.6 |
98 |
2 |
6 |
|
3.0 |
98 |
2 |
6 |
|
Capillary Voltage: |
1.0 V |
|
Desolvation Temp: |
400 °C |
|
Cone Gas Flow: |
Not used |
|
Desolvation Gas Flow: |
1000 L/Hr |
|
Collision Cell Pressure: |
2.6 x 10(-3) mbar |
|
MRM transition monitored, ESI+: |
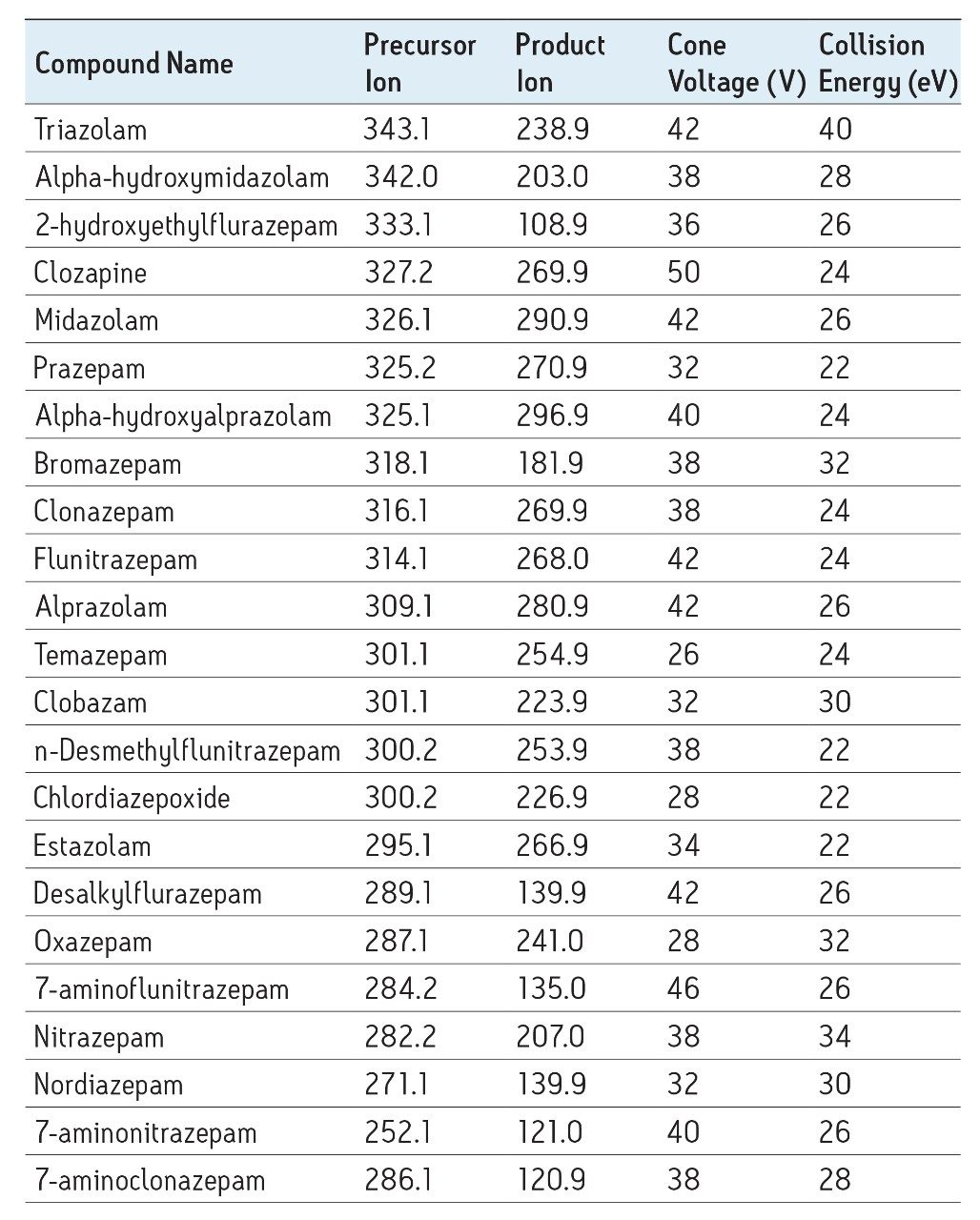
See Table 1 |
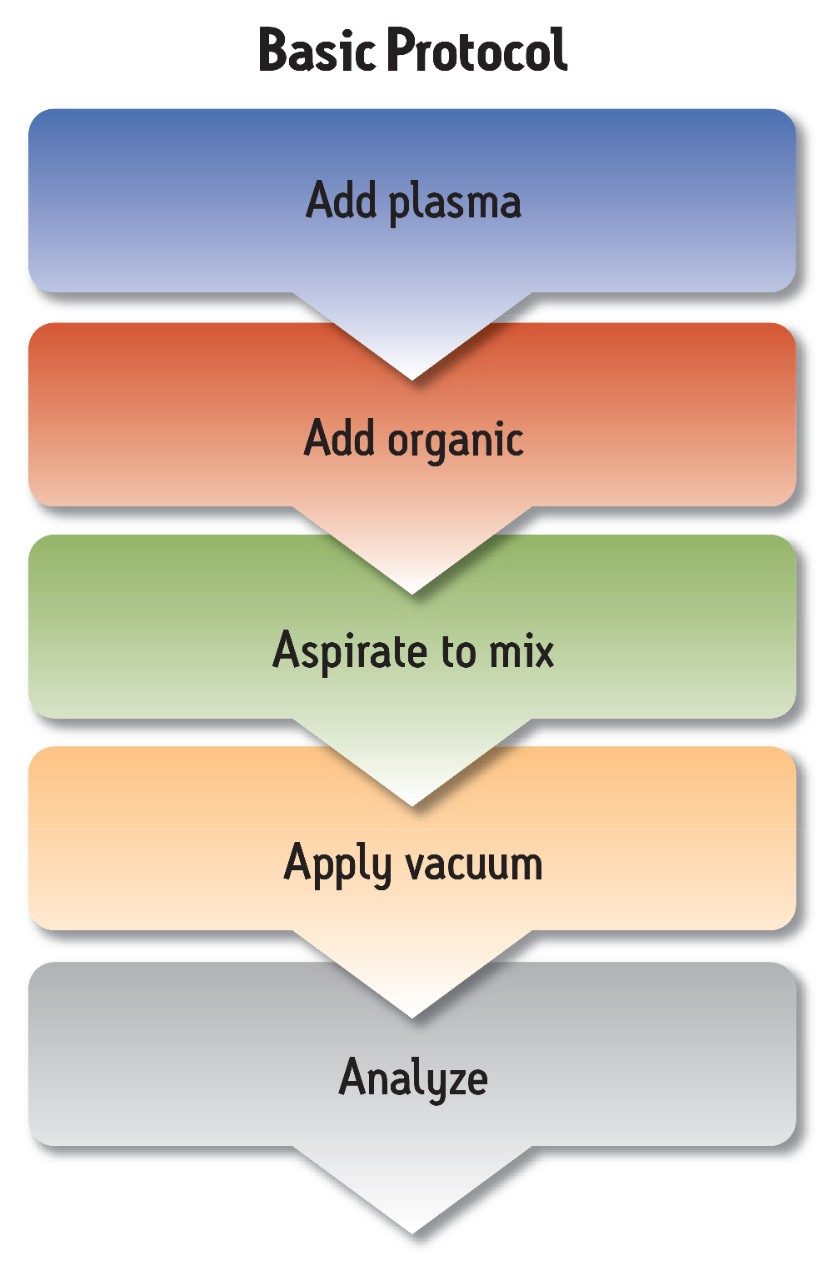
Sample preparation was performed using the standard protocol included with the Ostro 96-well plate (see Figure 2). Sample volume was 100 μL of plasma. This was extracted in the wells of the Ostro plate using 300 μL of 1% HCOOH in acetonitrile. Samples were mixed by aspiration. Vacuum was then applied to collect the eluates. Eluates were diluted 1:1 with water to ensure compatibility with the LC mobile phase and directly injected onto the LC-MS/MS system.
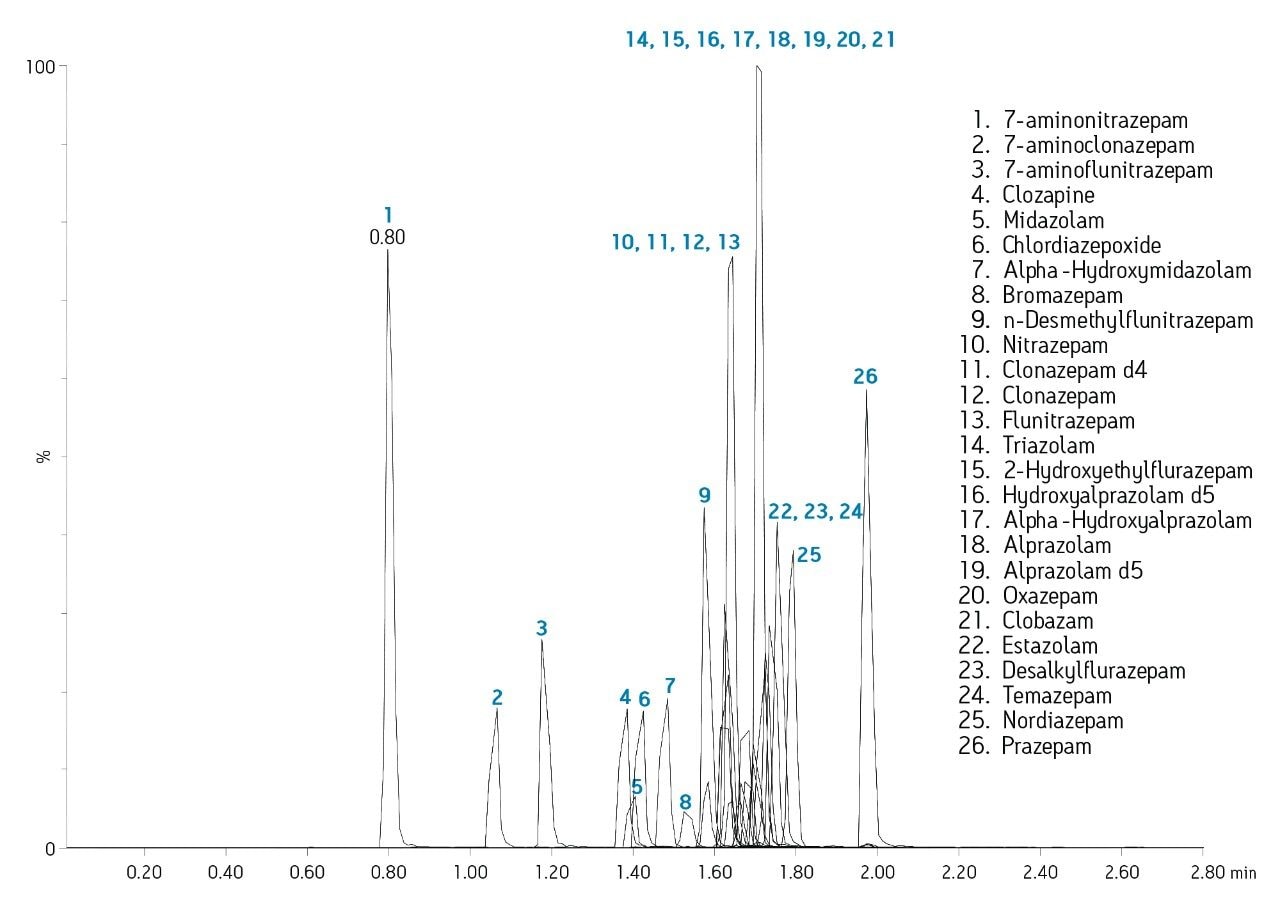
Separation of the 26 structural analogs (Figure 3) was achieved using a two-minute gradient at low pH with methanol. MS was performed in positive ion mode. Precursor and product ions were automatically optimized and an MS method automatically generated using IntelliStart software. A previous application note (720002569EN) by Rainville et al describes the capabilities of IntelliStart in detail.
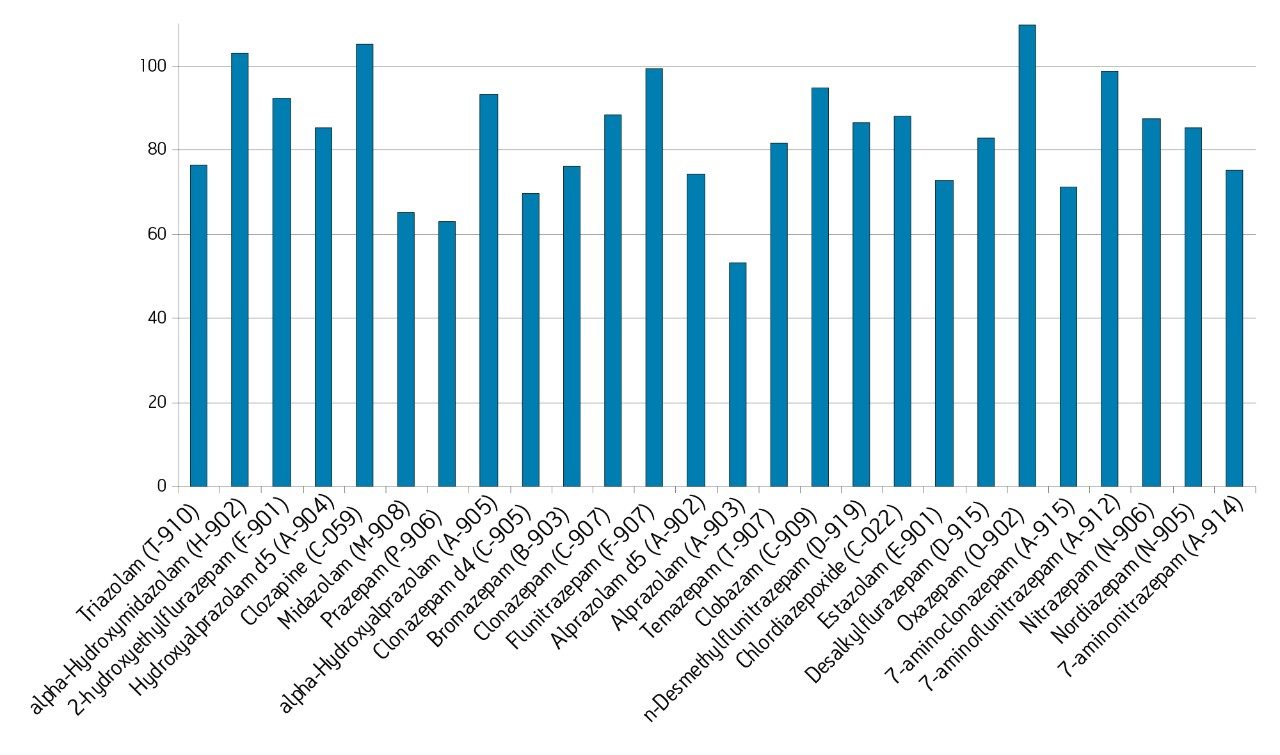
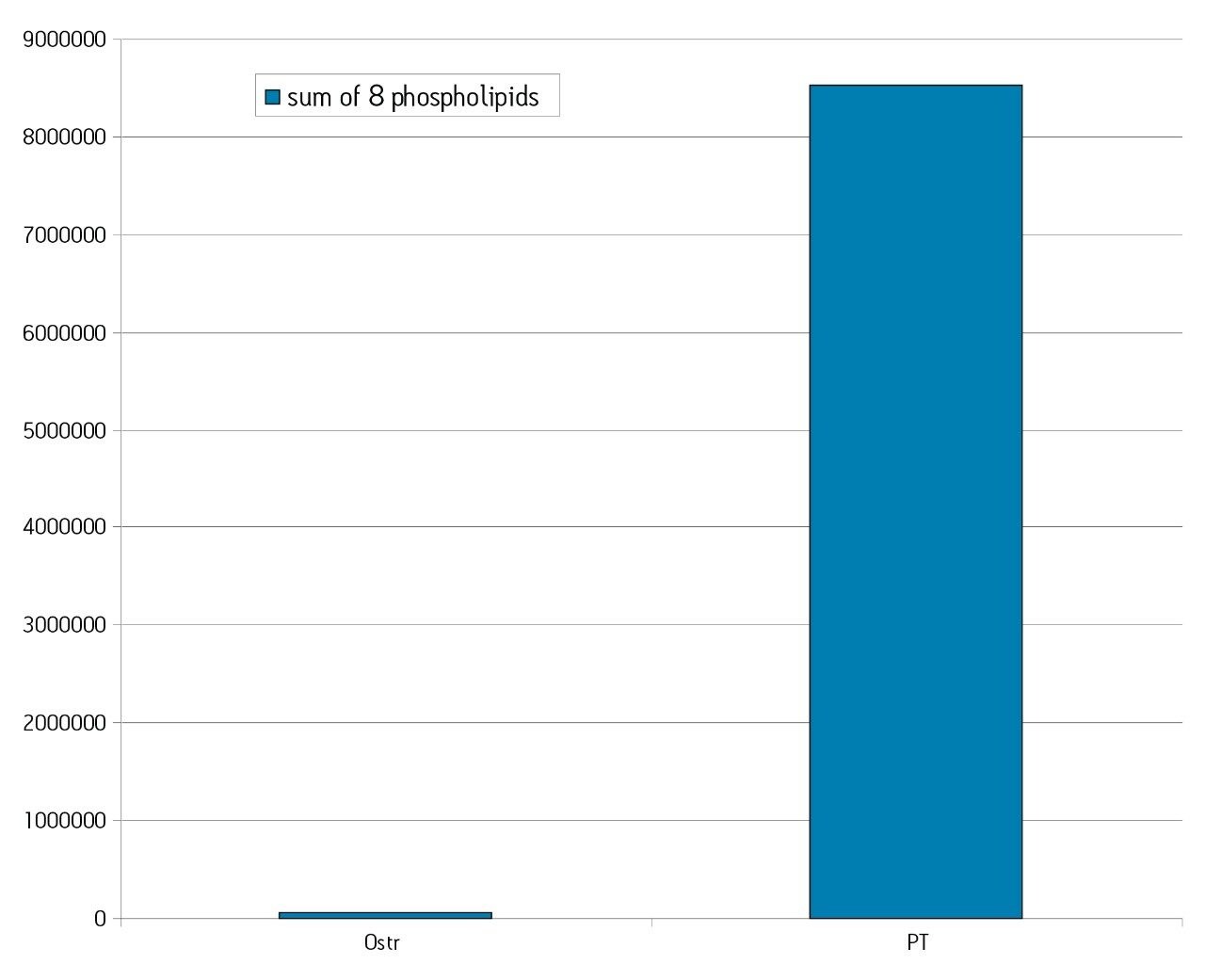
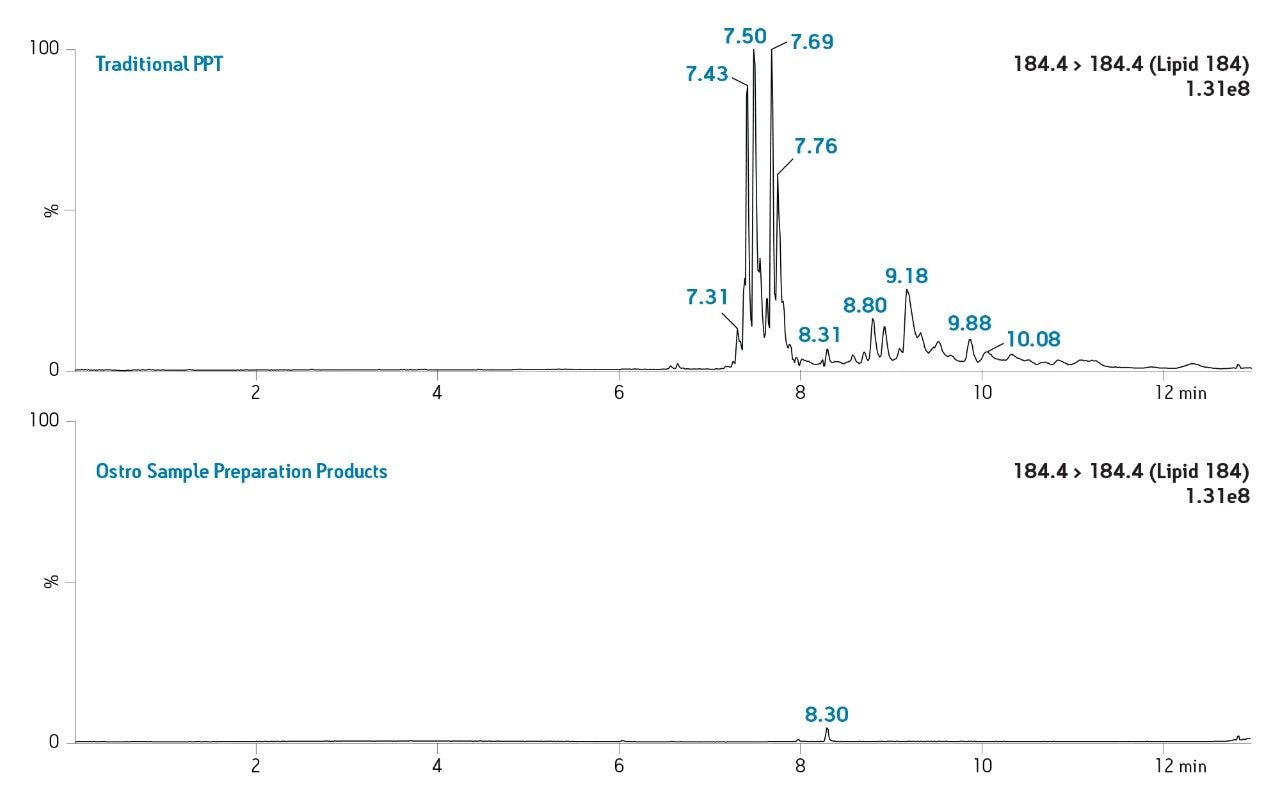
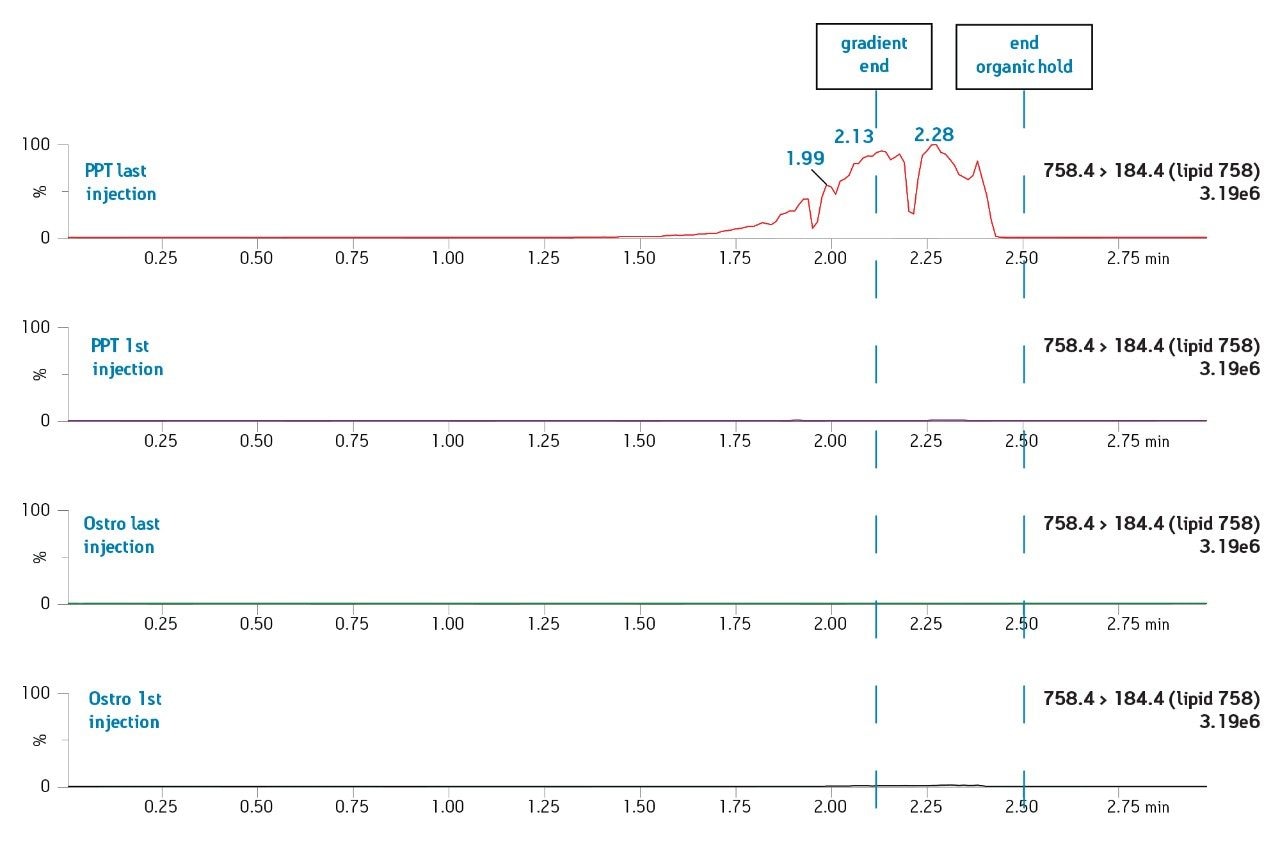
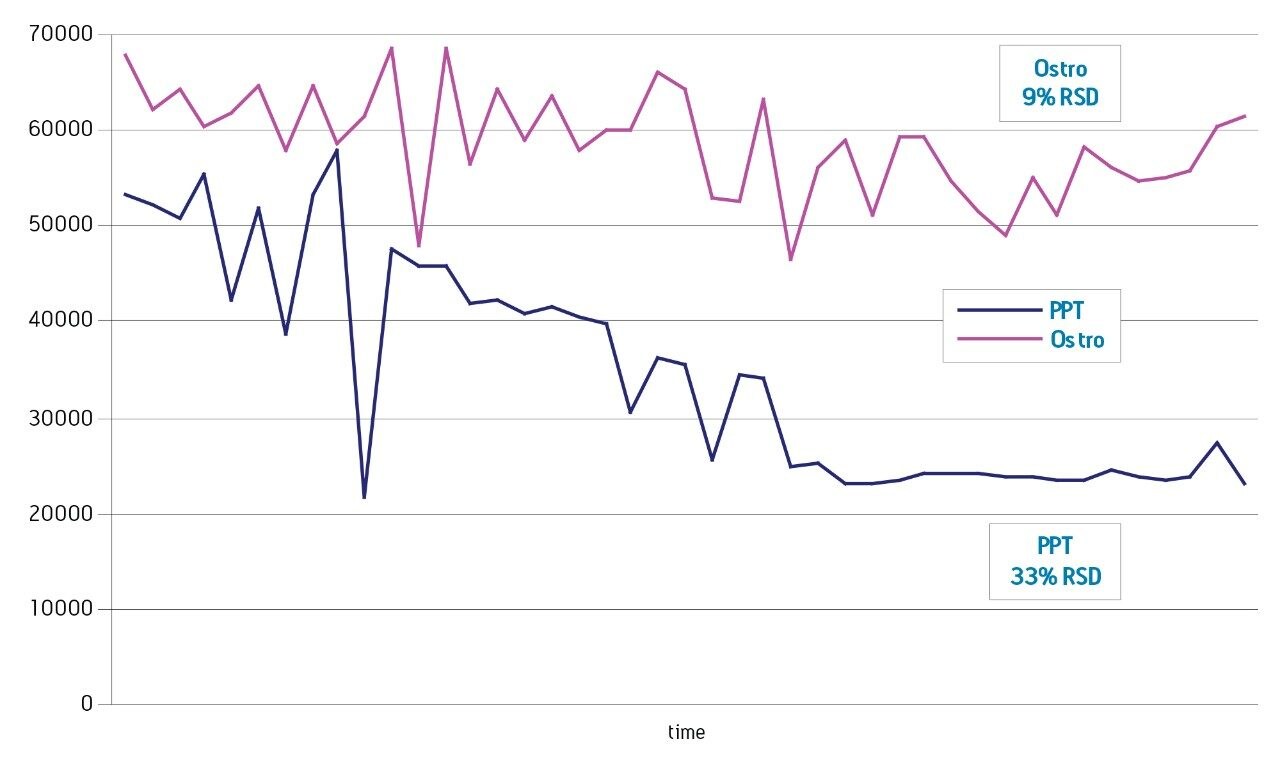
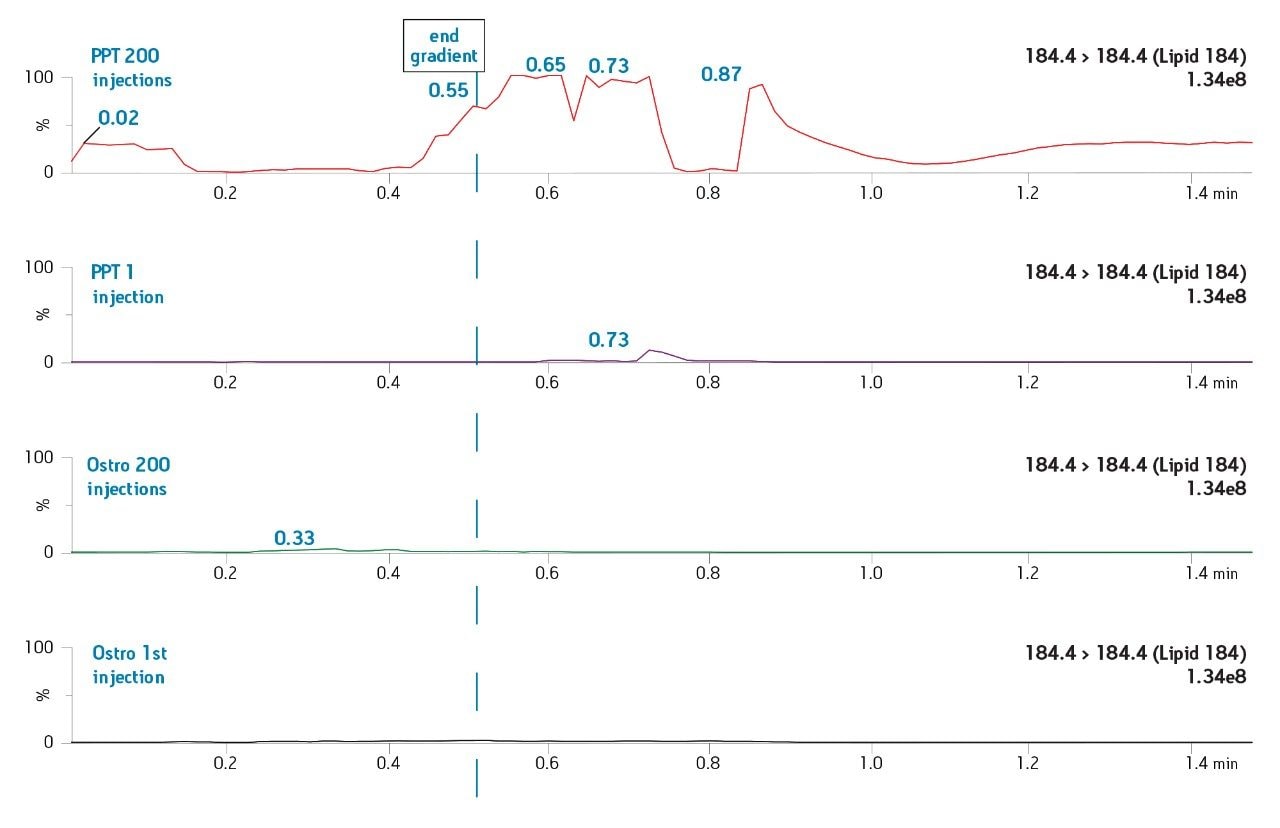
The Ostro 96-well plate was used to remove residual PLs prior to LC-MS/MS analysis. Utilizing the generic, simple protocol provided by the manufacturer (Figure 2), a group of analogous compounds were extracted. The resulting analysis demonstrated an average recovery of 86% for the group of structural analogs in plasma (Figure 4), which is equal to or better than traditional PPT. To assess PL removal, eight individual PLs were summed. Results showed that Ostro plates remove >99% of the 8 PLs relative to traditional PPT (Figure 5). In addition, the MRM transition, 184>184, was monitored to visually demonstrate the significant decrease in residual PLs using Ostro plates compared to traditional PPT (Figure 6). One of the primary reasons to eliminate PLs is to improve method robustness. Overnight runs of both Ostro plates and traditional PPT samples were carried out using the generic gradient and PLs were monitored continuously. Figure 7 shows the LC-MS trace for a representative PL at the beginning and end of the runs. When Ostro plates are used, the PL levels are negligible and no build-up occurs. When PPT is used, a significant amount of PLs are present and accumulate throughout the run. The result of this undesirable build-up is a continuous downward trend in area counts throughout the duration of the run (Figure 8). This in turn results in high signal variability relative to samples prepared with Ostro plates, 33% using PPT vs. 9% for Ostro samples. In addition, area counts decrease by 57% from the first injection to the final injection when PPT is used. In discovery bioanalysis, high throughput is of utmost importance. If one tries to increase throughput by shortening gradient time, the impact of residual PLs is further magnified. To demonstrate the negative effect PLs have on analytical throughput, the gradient time was decreased by half. Flow rate was increased and organic content was ramped from 50 to 98% in 0.5 minutes. 200 Ostro samples and 200 PPT samples were injected using the shorter gradient. The MRM transition 184>184 was monitored to reveal overall PL build-up and elution in the shortened gradient window (Figure 9). Using the 2 minute gradient, PLs elute within 0.2 minutes of the end of the gradient. Under the truncated gradient conditions PLs continue to elute for more than 1 minute after the end of the gradient and well into the reequilibration phase and beginning of the next injection. These resultant chromatograms demonstrate the inability to shorten gradient time with PPT due to PLs which continue to elute significantly after the gradient ends at 0.5 minutes. Overall, the Ostro plate allows for increased method robustness and reduced variability. Additionally both improved instrument uptime and the ability to significantly shorten run times are realized through the elimination of PLs, all of which are highly desirable in a discovery setting. Calibration curves from 1-500 ng/mL for each of the 26 structural analogs had a resulting average r2 value of 0.925, sufficient for discovery screens.
720004046, July 2011