Collision Cross Section Measurements of an Ensemble of Small Molecules Varying in m/z and Gas Phase Shape
This is an Application Brief and does not contain a detailed Experimental section.
Abstract
SYNAPT™ HDMS™ System can be used to differentiate flavanoid glycoside structural isomers, without the need for any LC separation or MS/MS fragmentation. We also report for the first time the determination of collisional cross-section (CCSs) of carbon C60 and C70 fullerene structures, which have traditionally only been analyzed on experimental non-commercial ion mobility instrumentation.
Introduction
In this technical note, we demonstrate how the SYNAPT HDMS System can be used to differentiate flavanoid glycoside structural isomers, without the need for any LC separation or MS/MS fragmentation. We also report for the first time the determination of collisional cross-section (CCSs) of carbon C60 and C70 fullerene structures, which have traditionally only been analyzed on experimental non-commercial ion mobility instrumentation.
We also compare the SYNAPT HDMS System T-Wave™-derived CCSs to the theoretically calculated CCSs using the open source code MOBCAL.1,2 Briefly, MOBCAL allows one to input a coordinate file (PDB File) of a structure of interest and obtain a theoretical CCS value. Using the Trajectory Method™1,2 algorithm, the orientationally averaged CCS is reported for fullerene C60 and C70; the antibiotic ampicillin and the polyamine spermine. The T-Wave ion mobility cell was calibrated using singly charged peptides of known collision cross-section.3

Experimental
MS and IMS conditions
|
MS system: |
SYNAPT HDMS |
|
Ionization mode: |
nanoESI + |
|
Capillary voltage: |
1 kV |
|
Cone voltage: |
35 V |
|
Trap CE: |
7 V |
|
Trans CE: |
4 V |
|
IMS bias : |
20 V |
|
Acquisition range: |
m/z 150–1000 |
|
Trap/Trans gas: |
SF6 |
|
IMS gas: |
He (3 mbar) |
|
IMS T-wave height: |
5.0 to 6.0 V |
|
IMS T-Wave speed: |
250 m/sec |
Results and Discussion
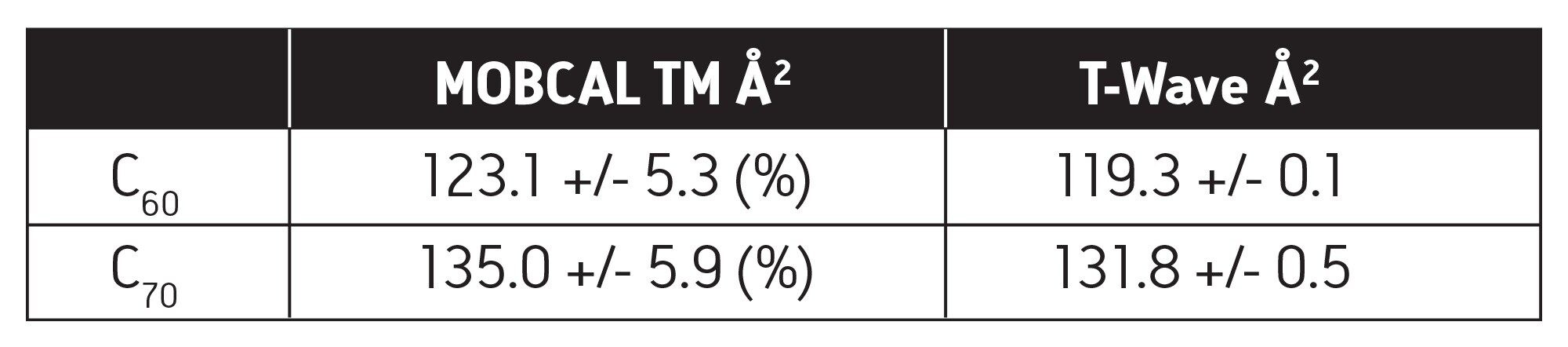
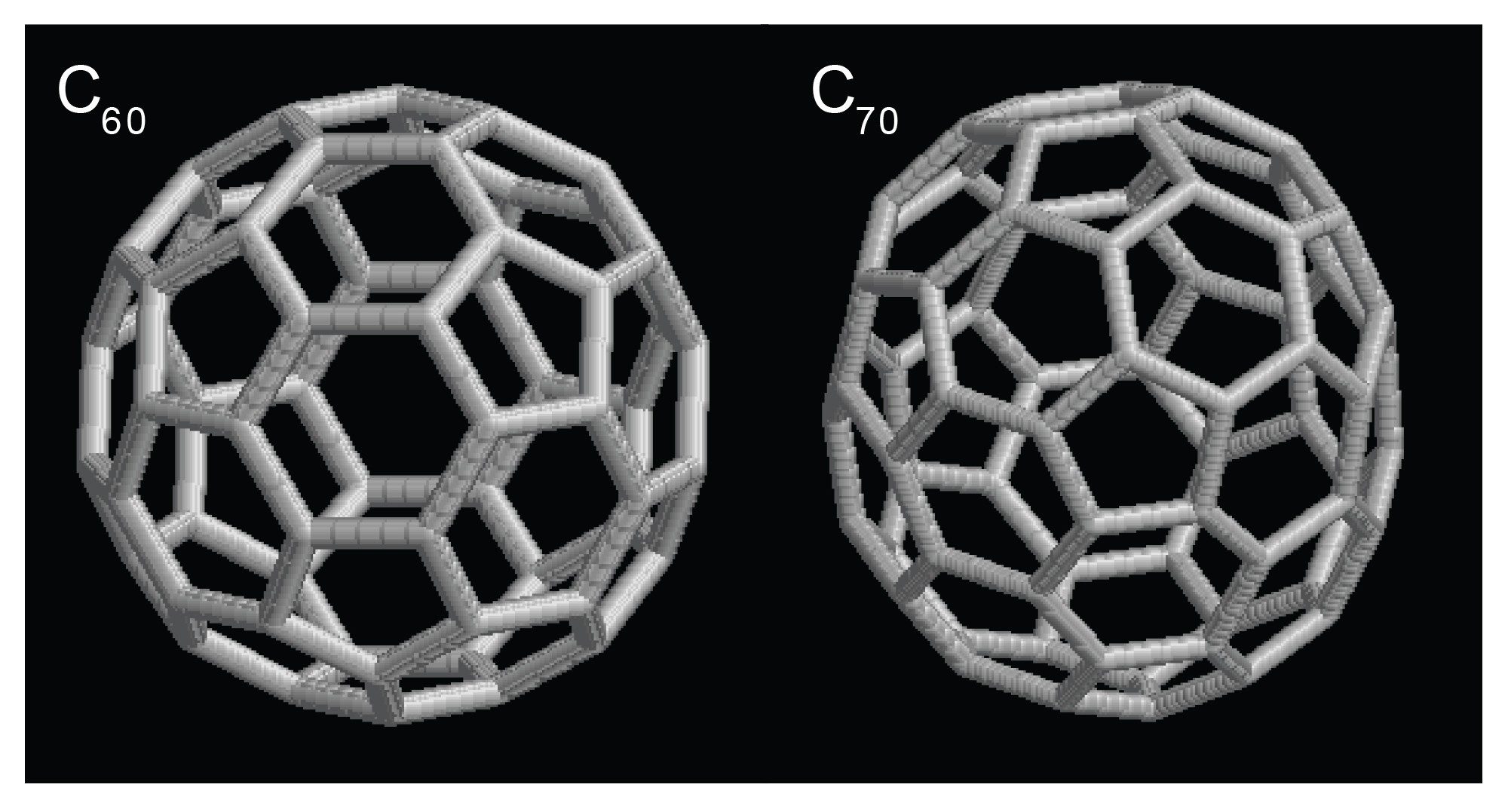
Carbon fullerenes are a family of molecules composed entirely of carbon and form a unique hollow structure. Historically, the shapes of inorganic clusters, such as fullerenes, have been studied by MALDI ionization on non-commercial, experimental ion mobility drift tube instruments4; these were the only instruments capable of making such measurements. Here, for the first time, we report the use of electrospray ionization and CCS determination of fullerene structures on a SYNAPT HDMS System, as shown in Table 1 and Figure 2. The CCS values derived from the T-Wave are identical to those obtained by the experimental drift tube instruments.4
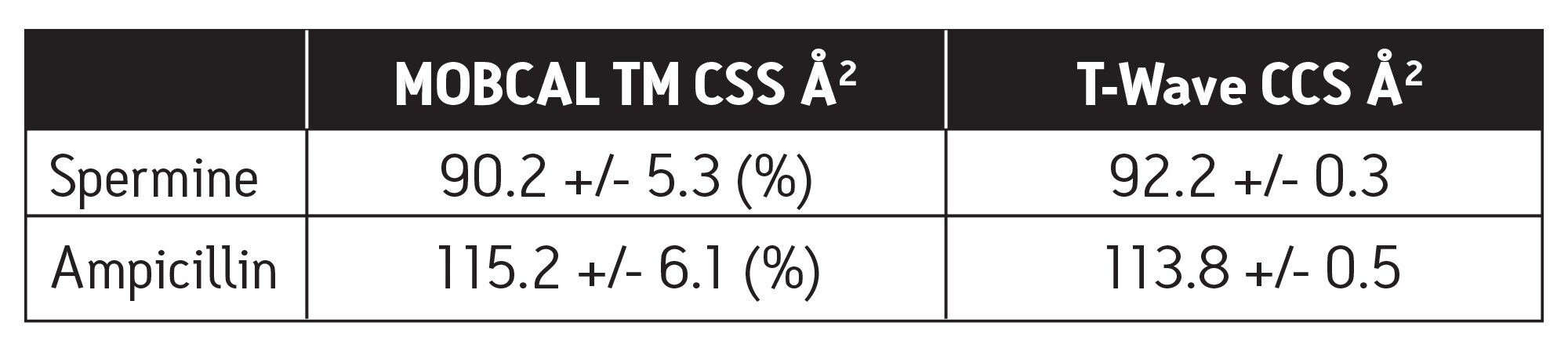
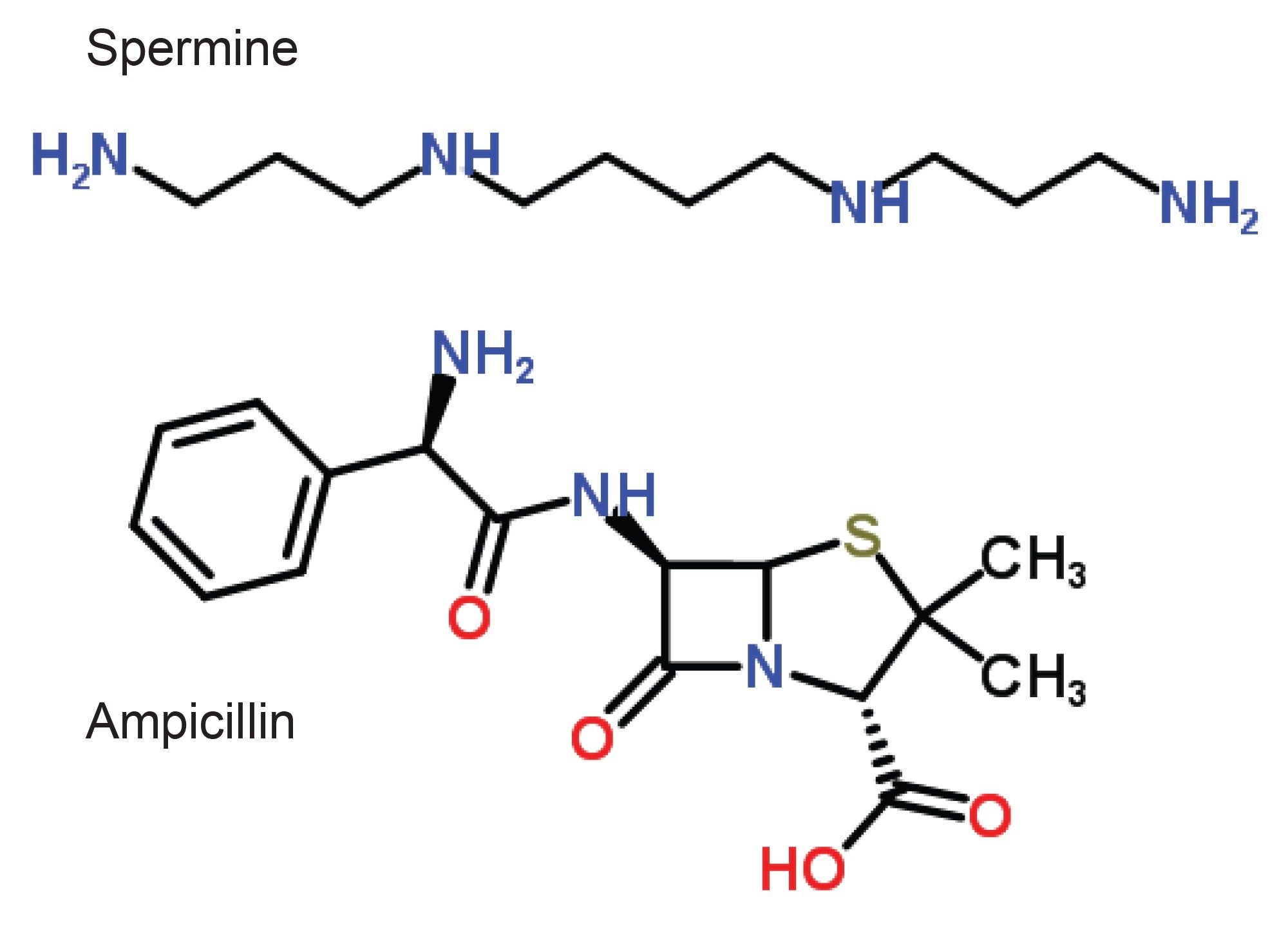
Utilizing the ion mobility capabilities of the SYNAPT HDMS System, one can now routinely measure gas phase shapes of molecules, such as spermine and ampicillin, as shown in Figure 3, and identify subtle shape differences in classes of compounds such as structural isomers, as shown in Figure 4.

Flavonoids are a large group of compounds built on a C6-C3-C6 flavone skeleton in which the three-carbon bridge between the phenol groups is cyclized with oxygen. The various classes of flavonoids are differentiated by the degree of unsaturation and oxidation of the three-carbon segment. Further differentiation is based on the number and nature of the moieties attached to the rings.
Flavonoids are often conjugated to a carbohydrate moiety. The type and number of monosaccharides and interglycosidic linkage, position, and type (O- or C-) of attachment of the glycan can vary, as shown in Figure 4. Flavonoids have previously been associated with a variety of biological activities such as antioxidative (radical scavenging), anti-inflammatory, and anti-depressant and are therefore of major interest to pharmaceutical companies.
Traditionally, LC/MS and MS/MS fragmentation have been used to differentiate structural isomers, since isomers have identical molecular mass. However, in certain cases, this has proved problematic.
In this Technical Note, we demonstrate how ion mobility can be used to differentiate flavanoid glycoside structural isomers, without the need for any LC separation or MS/MS fragmentation of the isomeric compounds.
In addition, we have shown for the first time, the ability to assign experimentally determined collisional cross sections to each of the structural isomers.
Figure 5 shows the different ion mobility drift times for the three luteolin structural isomers: luteolin 8 C-glucoside, luteolin 6 C-glucoside, and luteolin 7 O-glucoside. These drift time differences arise because the structural isomers have subtly different gas phase shapes and can therefore be separated based on their mobility differences.
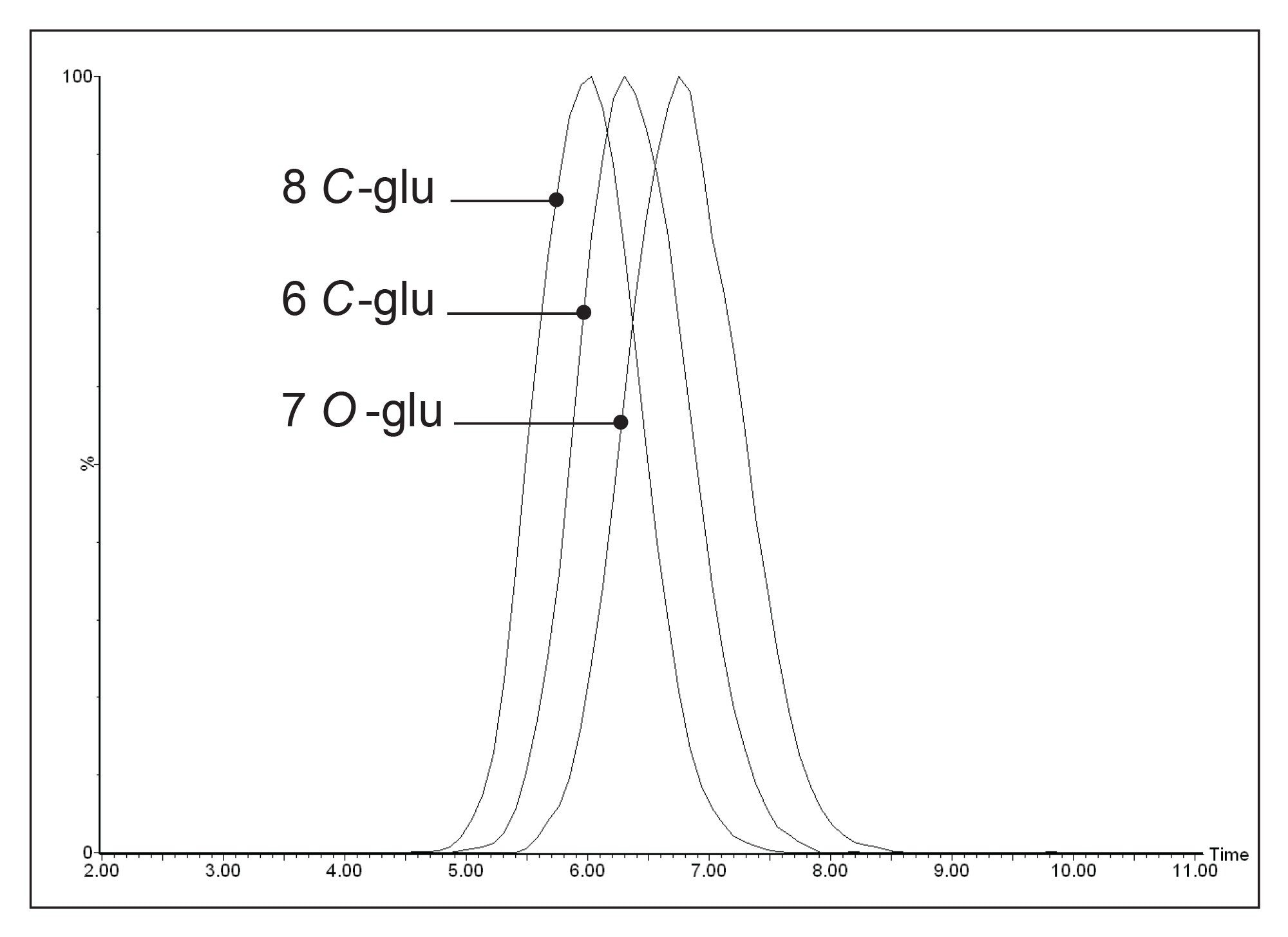
Table 3 shows the T-Wave derived collisional cross sectional areas for each of the luteolin glycoside structural isomers. Luteolin 8 C-glucoside has the smallest CCS and luteolin 7 O-glucoside has the largest CCS and, therefore, adopts the most elongated gas phase structure. This unique gas phase shape information cannot be obtained from MS data alone.
The technique of obtaining T-Wave derived CCSs of small molecules and comparing them to the theoretical CCSs, validates the method of T-Wave calibration and, thus, provides confidence when analyzing a compound whose molecular structure is unknown.
The use of ion mobility and accurate collision cross section determination of small structural isomers demonstrates and represents a powerful additional analytical technique in the world of bioactive small molecules and natural product analyses.
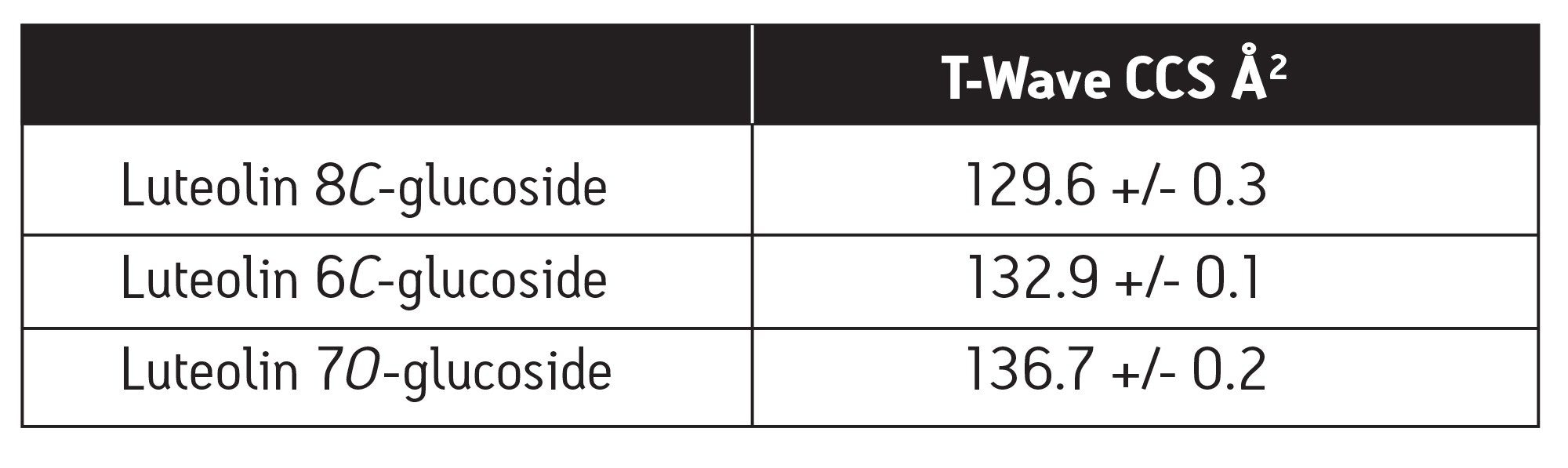
Table 3. T-Wave derived CCSs for three luteolin glucoside structural isomers. CCS standard deviations were obtained by acquiring IMS data at three T-Wave heights (5.0, 5.5, and 6.0 V).
Conclusion
■ Using singly charged peptides of known CCS to calibrate the T-Wave ion mobility cell, accurate CCSs of a variety small molecules can be determined. The collisional cross-sectionvalues obtained on the SYNAPT HDMS System are in excellent agreement with those derived by the experiment drift tube instruments.
■ The T-Wave derived CCSs for C60, C70, spermine, and ampicillin are in excellent agreement to the theoretically derived CCSs.
■ T-Wave ion mobility separation and CCS determination of three flavanoid glycoside structural isomers was achieved, demonstrating how the position of a single sugar unit can have profound impact on the gas phase shape of the ion. No molecular crystal or NMR structures were available for the isomeric structures.
■ Being able to identify shape differences in molecules is important, since changes in shape can result in significant differences in biological activity.
References
- Mesleh MF, Hunter HM, Shvartsburg AA, Schatz GC, Jarrold MF. Structural Information from Ion Mobility Measurements: Effects of the Long Range Potential, J Phys Chem, 1996, 100 (40), pp 16082–16086.
- http://www.indiana.edu/~nano/Software.html
- http://www.indiana.edu/~clemmer/Research/research.htm
- Dugourd PH, Hudgins RR, Clemmer DE, Jarrold MF. High-resolution Ion Mobility Measurements, Rev Sci Instrum 1997, 68, 1122.
- Pringle S, Worthington K, Bateman R. Travelling Wave Ion Propulsion in Collision Cells. Presented at the 51st ASMS Conference, Montreal, Canada 2003. The travelling wave device described here is similar to that described by Kirchner in U.S. Patent 5, 206, 506 (1993).
720002915, Aug 2022