
This is an Application Brief and does not contain a detailed Experimental section.
SDMS Vision Publisher serves as a reporting and authoring module incorporated as standard for NuGenesis SDMS and provides templates for Content Uniformity testing; whereas, NuGenesis SDMS acts as a data repository for analytical raw data as well as printed reports. A brief example will illustrate a Content Uniformity template for a tablet HPLC based assay. Other forms of Content Uniformity testing are also possible.
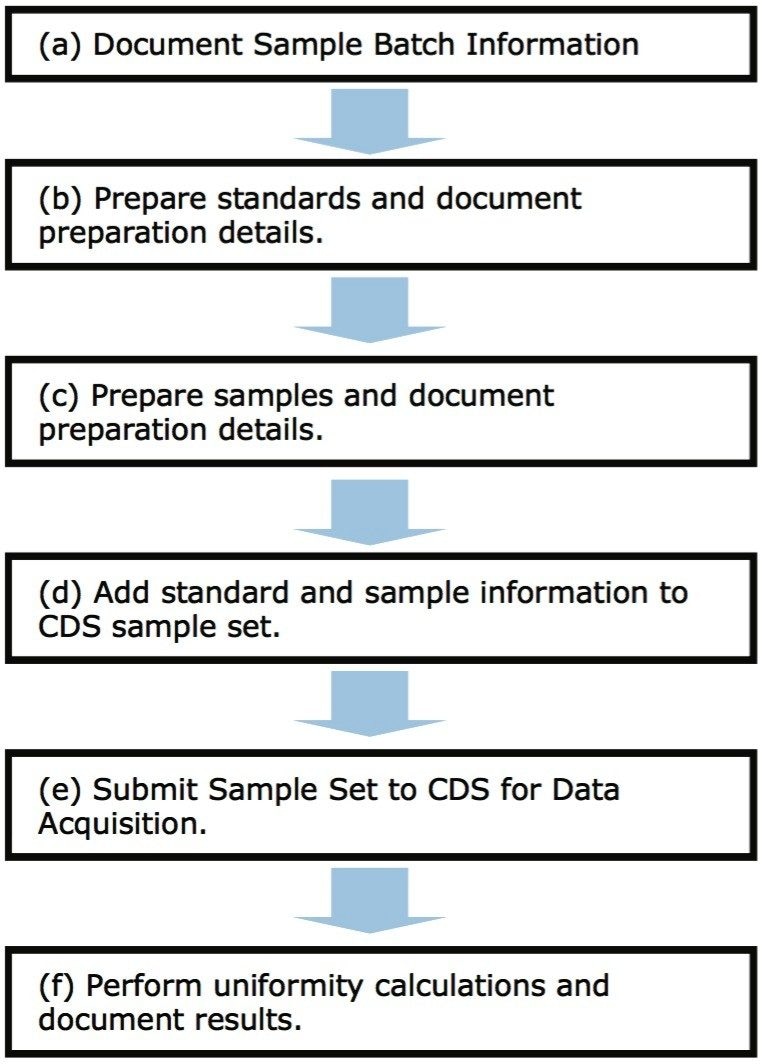
Releasing a pharmaceutical drug product to market may require a Content Uniformity assessment to ensure that the downstream consumer receives the recommended dosage of active pharmaceutical ingredient (API). Content Uniformity serves as one of several tests (other tests include appearance, average mass, dissolution, etc.) that are performed during manufacturing and that adhere to GMP guidelines. As defined by the USP, content uniformity tests may be performed on coated tablets, transdermal systems, soft gel capsules, packaged inhalations, single packaged solids, and suppositories.1-2 A typical Content Uniformity workflow is described in Figure 1 and consists of six primary steps. Each of these steps requires accurate documentation due to the adherence to GMP guidelines. Fortunately, the Content Uniformity workflow is straightforward and can make ready use of templates.
Content Uniformity studies employ a straightforward workflow that may be readily captured in a standardized template by using electronic report and authoring tools (see Figure 1 for workflow example). A standardized template both guides the analyst through the workflow and ensures reliable data capture without skipping necessary steps.3 Further benefits to recording Content Uniformity studies electronically include: searchable reports, electronic interface to analytical data systems (e.g., CDS, LIMS, balances), and electronic records that are 21 CFR Part 11 Compliant Ready.3
SDMS Vision Publisher serves as a reporting and authoring module incorporated as standard for NuGenesis SDMS and provides templates for Content Uniformity testing; whereas, NuGenesis SDMS acts as a data repository for analytical raw data as well as printed reports. A brief example will illustrate a Content Uniformity template for a tablet HPLC based assay. Other forms of Content Uniformity testing are also possible.
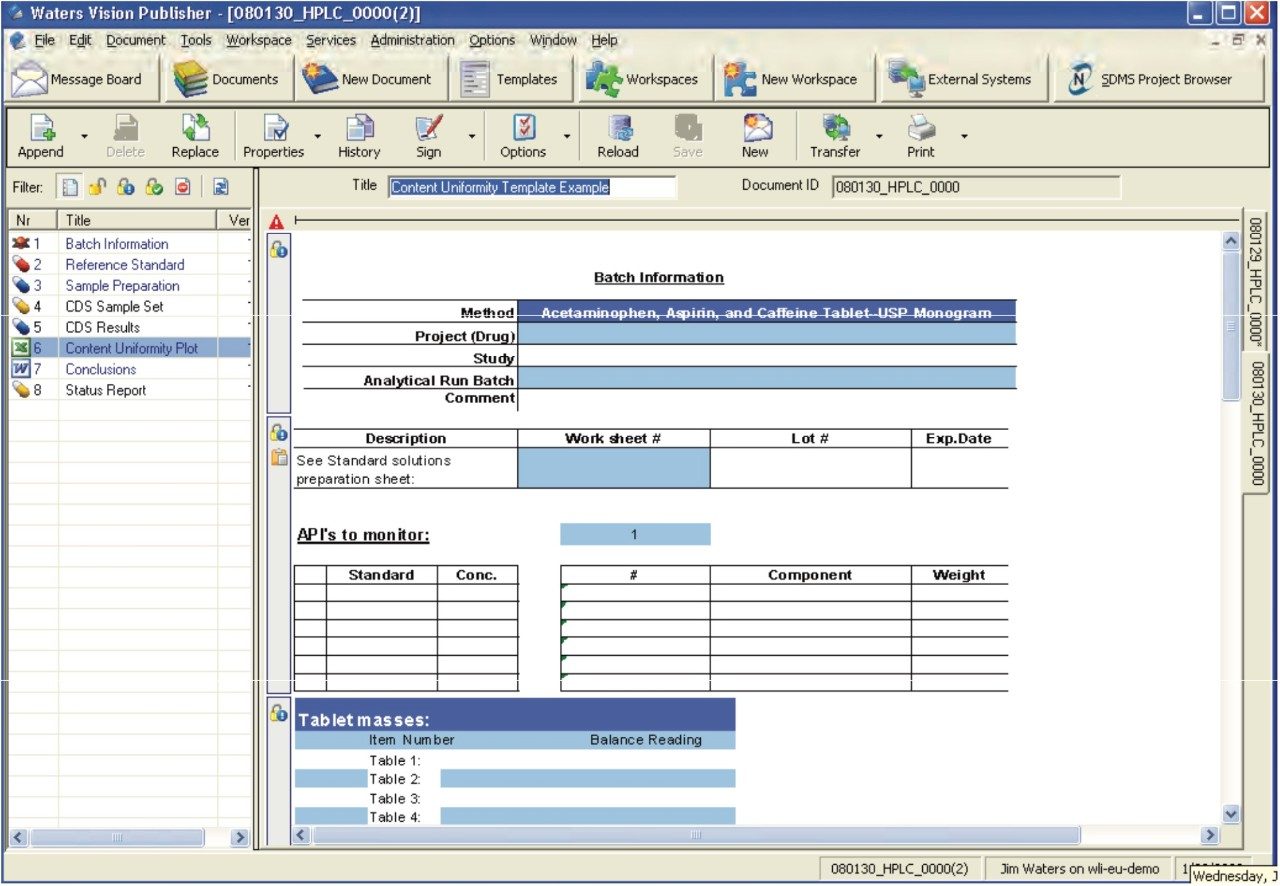
A typical tablet based content uniformity HPLC assay document requires four primary sections that include the reference standard preparation, the test sample preparation, the chromatographic system setup, along with the results and conclusions. The template highlighted in Figure 2 captures the aforementioned information as well as provides a convenient mechanism to automatically populate the Empower 2 sample set with reference standard and sample batch information, as well as automatic submission of the completed sample set to an Empower 2 System for acquisition. Subsequently, the results are then pulled back into the document from Empower 2 and the results are summarized.
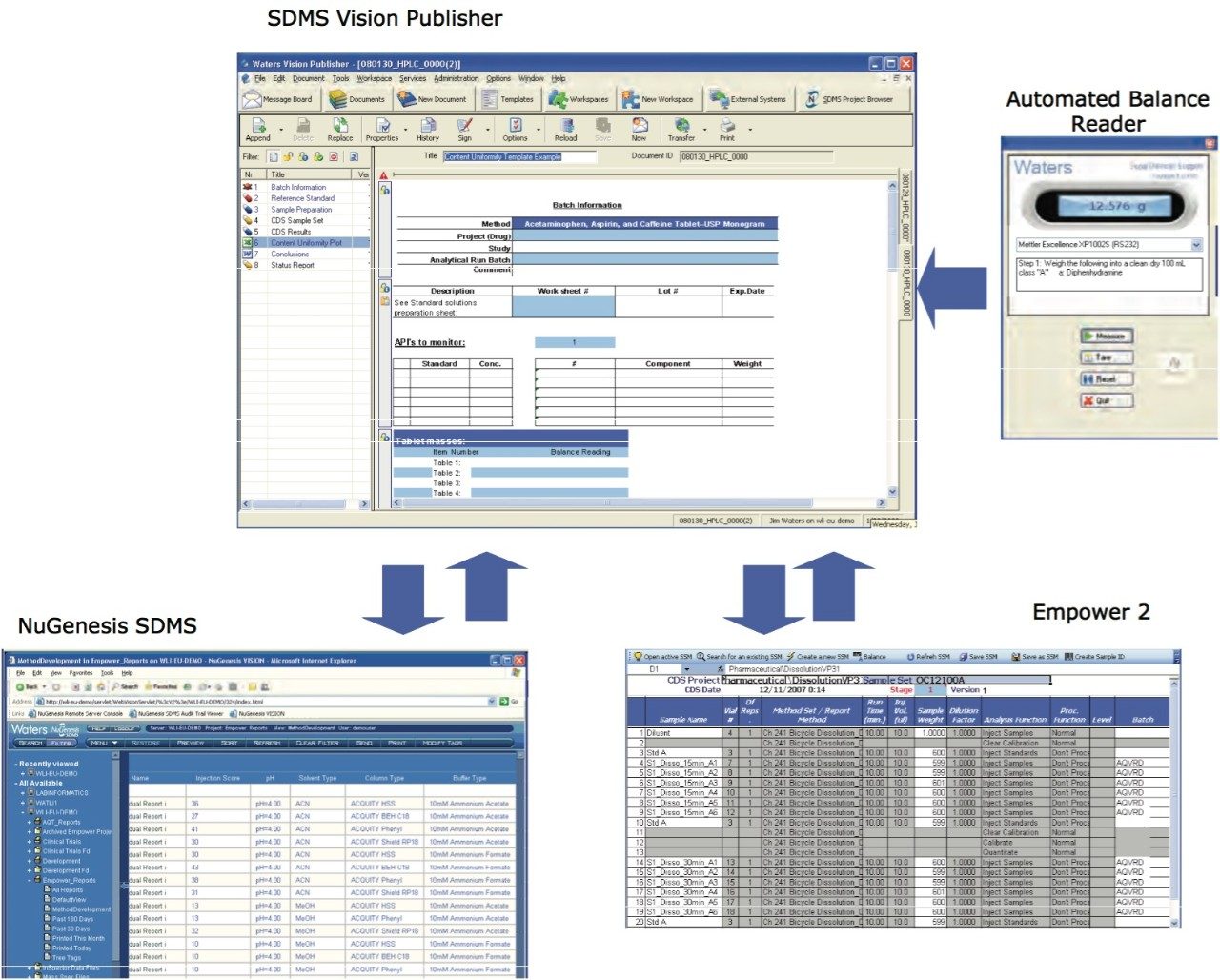
An electronic document provides a convenient means to find data and records by allowing online searching. It also empowers the user to do more than what was possible with a paper document because it can interface with other electronic data sources such as balances, CDS, LIMS, etc.3 For example, it can automatically record balance measurements during the standard and sample preparation stage; hence, it reduces the risk of transcription errors (Figure 3). Additionally, assay method reports can be stored in NuGenesis SDMS and can be easily retrieved and added to the content uniformity document, reducing the analyst’s documentation burden (Figure 3). Finally, the HPLC sample set can be automatically populated, uploaded to Empower 2, and the results returned again to the Content Uniformity document (this is also possible with other Chromatography Data Systems).
Content Uniformity is a critical test required before releasing a drug product to market. The process comprises a multi-step workflow where accurate documentation of results is an absolute necessity. The Waters Informatics suite provides an integrated solution that can dramatically streamline workflow, increase laboratory productivity, and facilitate product development and release.
720002554, April 2008