An approach to improving separations by transferring methods from HPLC to UPLC is outlined.
Ultra performance liquid chromatography (UPLC) is a technological breakthrough in separation science. Significant gains in speed, sensitivity, and resolution are enabled by UPLC. To harness the power of UPLC in analytical laboratories, many HPLC methods can easily and routinely be transferred to UPLC by using readily available tools. In this application, we will transfer a method for the separation of caffeic acid derivatives found in echinacea purpurea from HPLC to UPLC.
|
Column: |
XTerra MS C18 4.6 x 150 mm, 5 µm |
|
Mobile phase A: |
0.1% TFA in water |
|
Mobile phase B: |
0.08% TFA in acetonitrile |
|
Flow rate: |
1.0 mL/min |
|
Injection volume: |
10 µL |
|
Sample concentration: |
100 µg/mL |
|
Column temperature: |
40 °C |
|
Detection: |
UV @ 330 nm |
|
Sampling rate: |
5 pts/s |
|
Time constant |
1.0 |
|
Instrument: |
Waters Alliance 2695 Separations Module with 2996 PDA |
In this case, the HPLC column is an XTerra MS C18 Column. We can use the Waters Reversed-Phase Column Selectivity Chart to choose the ACQUITY UPLC chemistry that is closest in selectivity to the HPLC column. This tool is available at www.waters.com/selectivitychart. Using the tool, the ACQUITY UPLC BEH C18 Column is closest in selectivity. The HPLC column dimensions were 4.6 x 150 mm, 5 µm. To maintain the same resolution, we select a UPLC column that has a similar column length to particle size ratio (L/dp) as the HPLC column. Thus, we selected a 2.1 x 50 mm, 1.7 µm UPLC Column.
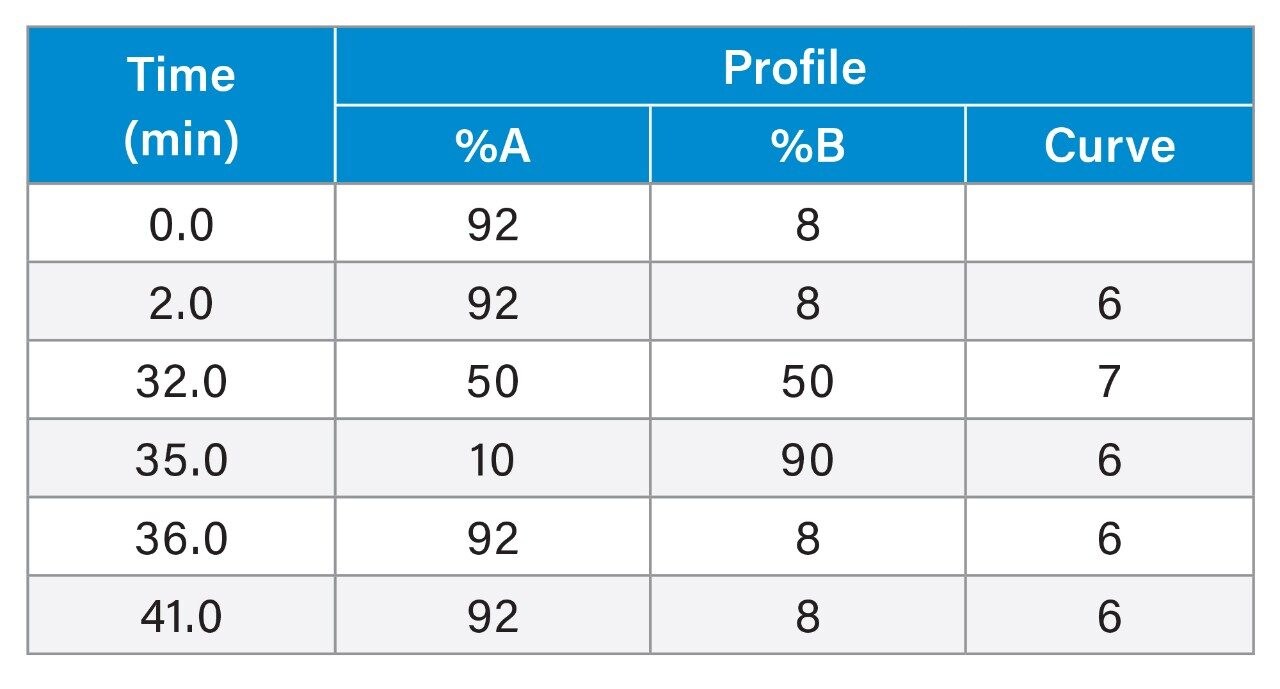
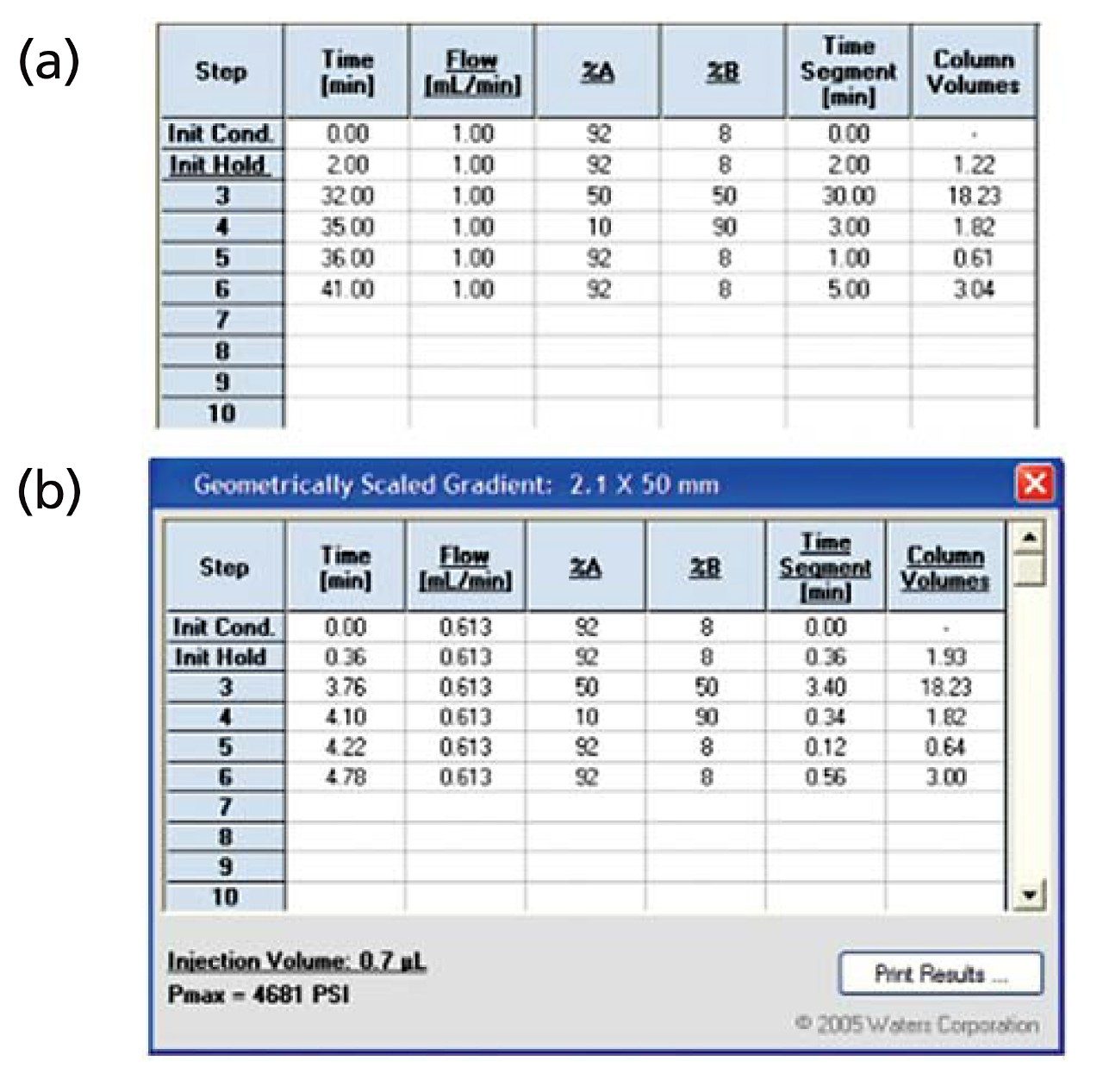
To scale the separation from HPLC to UPLC a series of calculations are performed to scale the flow rate, injection volume and gradient table. To ensure accurate and quick scaling, the ACQUITY UPLC Columns Calculator handles all of the appropriate scaling calculations. We input the HPLC conditions into the calculator (as shown in Figure 1A) and the UPLC conditions are automatically displayed (as shown in Figure 1B).
|
Column: |
ACQUITY UPLC BEH C18 2.1 µ50 mm, 1.7 µm |
|
Mobile phase A: |
0.1% TFA in water |
|
Mobile phase B: |
0.08% TFA in acetonitrile |
|
Flow rate: |
0.613 mL/min |
|
Injection volume: |
0.7 µL |
|
Sample concentration: |
100 µg/mL |
|
Column temperature: |
40 °C |
|
Detection: |
UV @ 330 nm |
|
Sampling rate: |
20 pts/s |
|
Time constant: |
0.2 |
|
Instrument: |
ACQUITY UPLC with ACQUITY 2996 PDA |
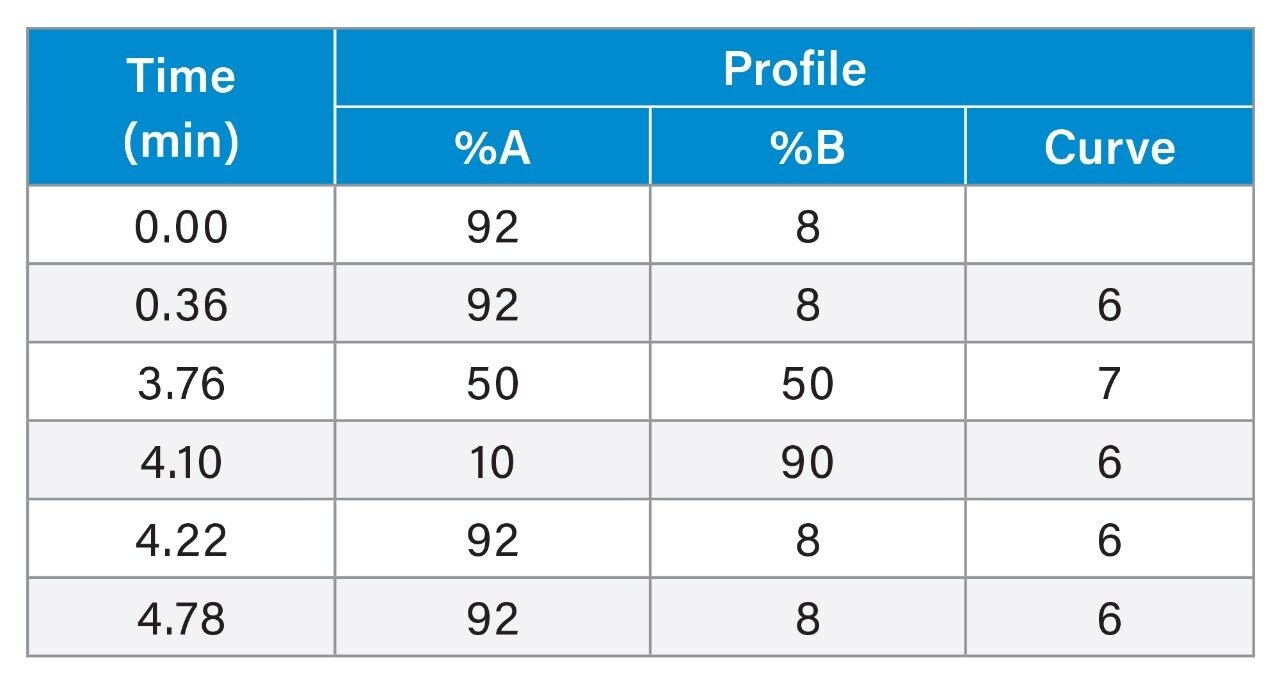
The original HPLC method had a cycle time of 41 min and resolution values as listed in Table 1. By using the Waters Reversed-Phase Column Selectivity Chart and the ACQUITY UPLC Columns Calculator, we successfully transferred the method to UPLC. The new method has very similar resolution as listed in Table 1 and a cycle time of only 4.78 min – an 8.5x improvement in cycle time.
For improvements in speed, resolution, and sensitivity, HPLC methods can be successfully transferred to UPLC by using the available electronic tools. These separation improvements translate into significant cost reductions in the analytical laboratory.
WA43218, June 2006