
This is an Application Brief and does not contain a detailed Experimental section.
This application brief compares the sensitivity of the Quattro Premier with that of the the Quattro Premier XE Tandem Quadrupole Mass Spectrometer in negative ion mode, using 2 test compounds: the non steroidal anti-inflammatory (NSAID) drug Ibuprofen, and the steroid Prednisolone.
As part of the pharmaceutical development process, it is a regulatory requirement to determine the toxicity and efficacy of a candidate compound prior to its release to market. These safety assessment and clinical trial studies require monitoring for compound exposure. The key criteria in these analyses are sensitivity, throughput and robustness. The accepted technology for these analyses is LC-MS/MS operating in MRM mode. Waters ACQUITY UPLC System technology brings faster analyses with lower levels of detection. UPLC offers improved resolution over HPLC, rapid gradient analysis with optimized injection cycles. To complement, the Waters Micromass Quattro Premier XE Mass Spectrometer has been specifically designed for unsurpassed productivity and sensitivity, applying enhanced electronics and a new detector to yield significantly enhanced performance.
|
LC system: |
ACQUITY UPLC System |
|
Column: |
ACQUITY UPLC BEH C18 2.1 x 50 mm, 1.7 μm |
|
Flow rate: |
0.6 mL/min |
|
Mobile phase: |
A = 10 mM NH4OAc in 20% MeOH, pH 5.0 B = 10 mM NH4OAc in 80/20 MeOH/ACN |
|
Time (min) |
%A |
Curve |
|---|---|---|
|
0.0 |
95 |
6 |
|
0.8 |
5 |
6 |
|
1.0 |
5 |
6 |
|
3.0 |
55 |
1 |
|
MS system: |
Quattro Premier and Quattro Premier XE Tandem Quadrupole Mass Spectrometers |
|
ESI Capillary voltage: |
3.0 Kv |
|
Source temp.: |
130 °C |
|
Desolvation temp.: |
380 °C |
|
Desolvation gas flow: |
800 L/hr |
|
Cone gas flow: |
50 L/hr |
|
Ionization mode: |
ESI- |
|
Dwell time: |
100 ms |
|
MRM transitions: |
Prednisolone = 359.5→329.1 Ibuprofen = 205.2→161.2 |
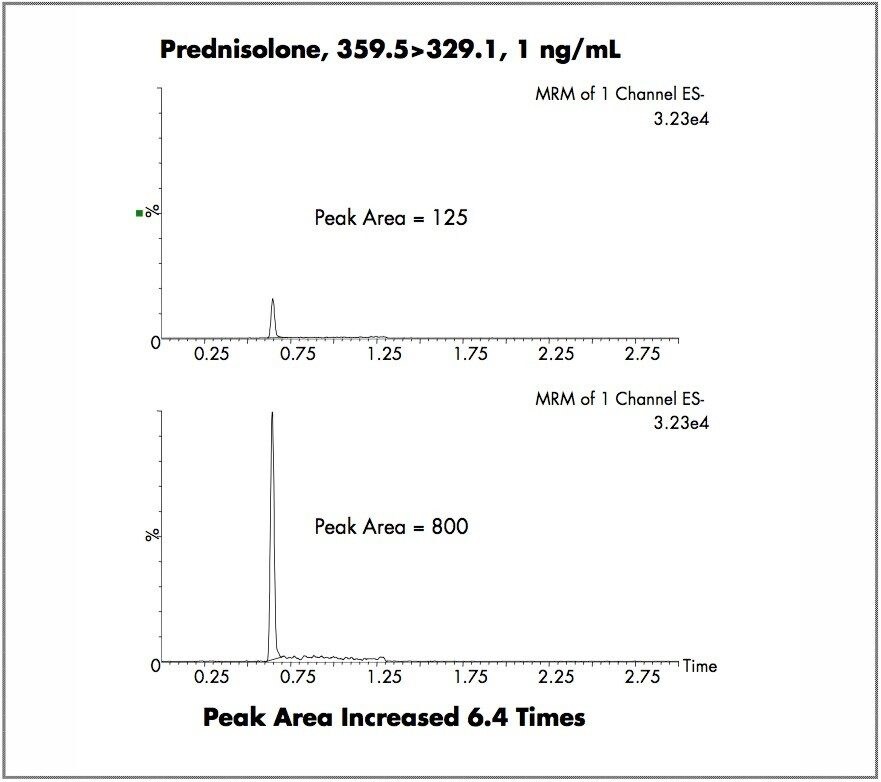
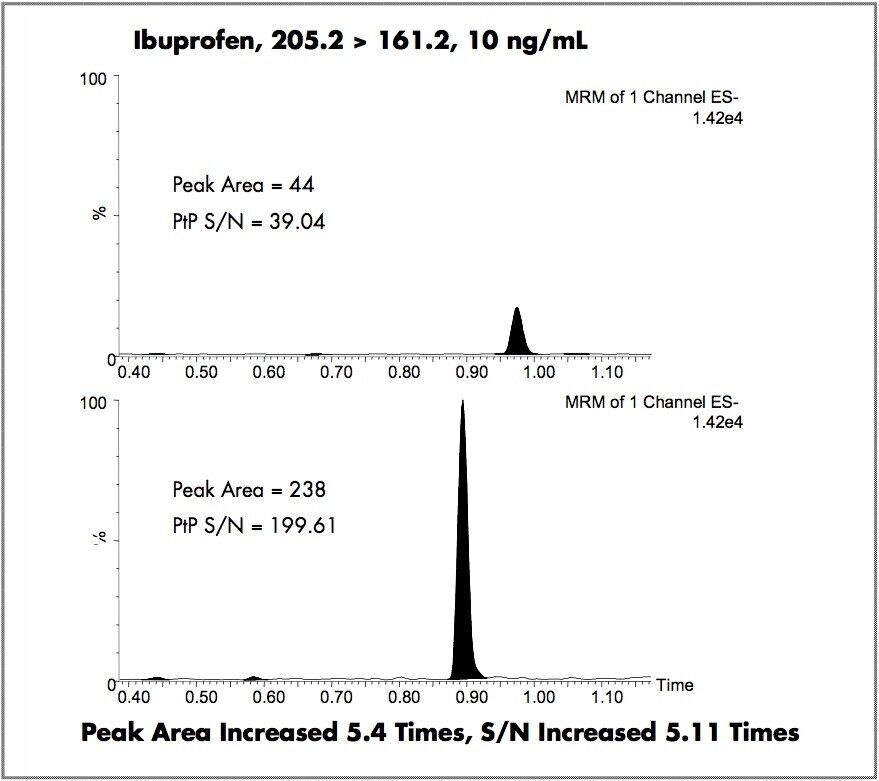
The sensitivity of the Quattro Premier XE in negative ion mode was compared to that of the Quattro Premier. The data obtained from the analysis of the steroid prednisolone is displayed in Figure 1, using the MRM transition of 359.5→329.1. As demonstrated, with the same on-column loading and the same MRM parameters, a peak area increase of 6.4-fold was observed. In the second experiment, the NSAID compound ibuprofen was used as the test analyte. The results obtained are displayed in Figure 2. Here again we can see that with the same on-column loading and MS/MS parameters, a peak area increase of 5.4-fold was observed and the signal-to-noise ratio was increased by a factor of 5.
The Quattro Premier XE Tandem Mass Spectrometer provides unmatched sensitivity in both positive and negative ionization modes. Additionally, the ACQUITY UPLC System produces the ultimate in chromatographic throughput and sensitivity, generating sharp peaks, 2 seconds at the base, with run times on the order of 1 minute. Combining these results in a premium analytical platform for increased data quality and productivity in quantitative bioanalysis.
720001412, November 2005