For research use only. Not for use in diagnostic procedures.
This is an Application Brief and does not contain a detailed Experimental section.
The aim of this application brief was to consolidate the assays for cyclosporin A and tacrolimus in a single analytical method. This will provide a very powerful tool as combined therapy gains popularity in an effort to reduce toxicity.
The limit of detection is below the therapeutic range of interest
Cyclosporin A is an immuno-suppressive agent used to control rejection in transplant patients caused by antigenic differences remaining after tissue-typing and donor-recipient matching. Transplant centres have found that monitoing the blood concentration of cyclolsporin is an essential component of patient management, as higher levels of the drug are known to be severely nephrotoxic.
In previous work, (Application Brief 19) we examined the quantification of another immuno-suppressive drug, tacrolimus (FK 506) using ascomycin as an internal standard. The aim of this study was to consolidate the assays for cyclosporin A and tacrolimus in a single analytical method. This will provide a very powerful tool as combined therapy gains popularity in an effort to reduce toxicity.
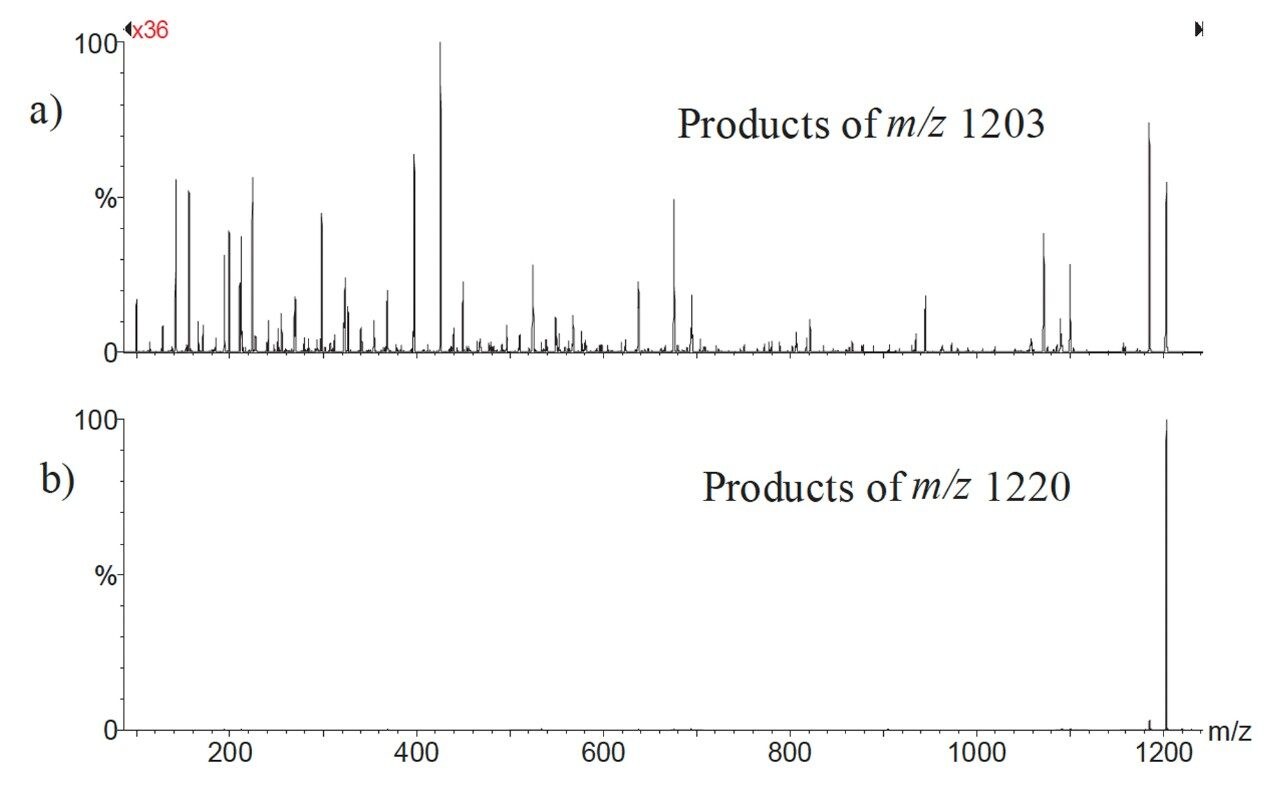
A Quattro LC Tandem Mass Spectrometer fitted with a Z SPRAY ion source was used for all analyses. The instrument was operated in electrospray positive ionisation mode and was coupled to a Waters 2790 Alliance HT HPLC System. All aspects of system operation and data acquisition were controlled using MassLynx NT v3.4 Software with automated data processing using the MassLynx Quantify program. Previous studies have shown that maximum sensitivity for cyclosporin A can be obtained by monitoring fragmentation of the ammonium adduct ([M+NH4]+) to the protonated molecule. A comparison of the fragnmentation of the protonated and the ammoniated molecules are shown in Figure 1. As can be clearly seen, the fragmentation of the ammoniated species is much more specific (and therefore much more sensitive) that the fragmentation of the protonated molecule. A solution of Cyclosporin A (10 μM) in mobile phase (vide infra) was infused into the ion source, and the cone voltage optimised to maximise the intensity of the [M+NH4]+ precursor (parent) ion (m/z 1220.0). The collision energy was then adjusted to optimise the signal for the most abundant product ion (m/z 1203.0).
The process was repeated for the tacrolimus analogue, ascomycin (FR900520) which was used as an internal standard to correct for losses during sample processing. Precipitant was prepared by dissolving ascomycin in acetonitrile at a concentration of 45 μg/L. EDTA blood (100 μL) was placed in a 1.5 mL Eppendorf tube and exactly 2 volumes of precipitant added to give a final equivalent concentration of 30 μg/L Ascomycin. Samples were vortex mixed for 30 seconds before centrifuging at 13000 rpm for 5 minutes. The supernatant was transferred to a clean tube and injected into the LC-MS/MS system.
An isocratic HPLC method was used for on-line sample clean-up using a Spherisorb CN Column (30 mm x 4.6 mm, 5 μm) and 65% aqueous acetonitrile containing 2 mM ammonium acetate and 0.1% (v/v) formic acid. All the compounds of interest (cyclosporin A, ascomycin and tacrolimus) eluted in less than 1 minute, and a cycle time of 2 minutes (injection to injection) was easily achieved.
Although the fragmentation of cyclosporin seems very simplistic (loss of ammonia from an ammoniated species) the results show that there is no interference from endogenous compounds. Figure 2 shows the reconstructed ion chromatograms for three concentrations of cyclosporin in whole blood. Note that the spectra are normalised to the highest peak in the vertical pairings.
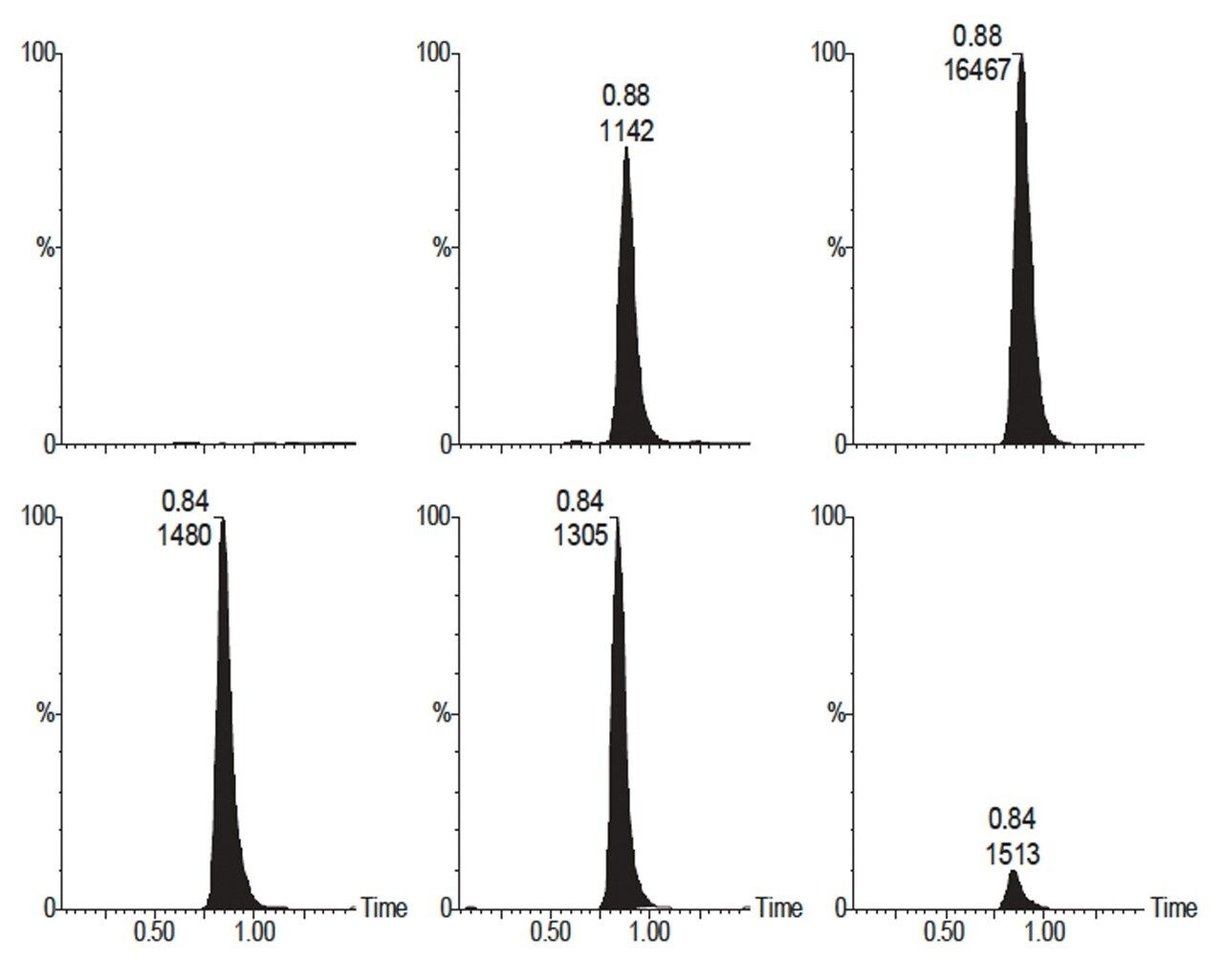
One facet of the elution of the compounds from the CN column is the differences in peak shape observed between the cyclosporin and the ascomycin. The increased retention of the larger cyclosporin leads to broadening of the peak, and means that a simple mass spectral peak intensity ratio method of quantification is not viable. Quantification was performed by integrating the area under the extracted ion chromatograms for cyclosporin A and ascomyin, and generating a standard curve using whole-blood calibrators.
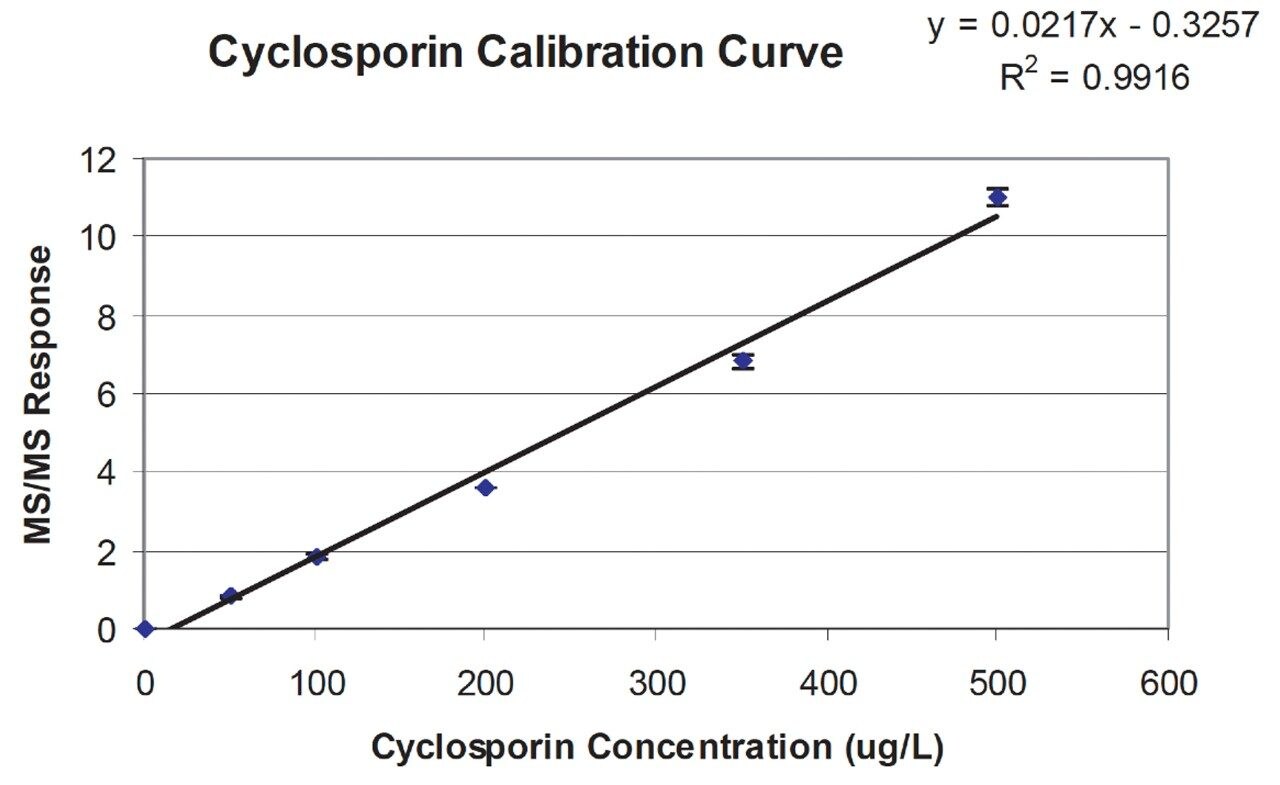
The precision of the LC-MS/MS analysis of the extracted samples was >95% over 50 injections. The calibration curve was linear (Figure 3) and showed a good correlation with the stated values (r2 = 0.992).
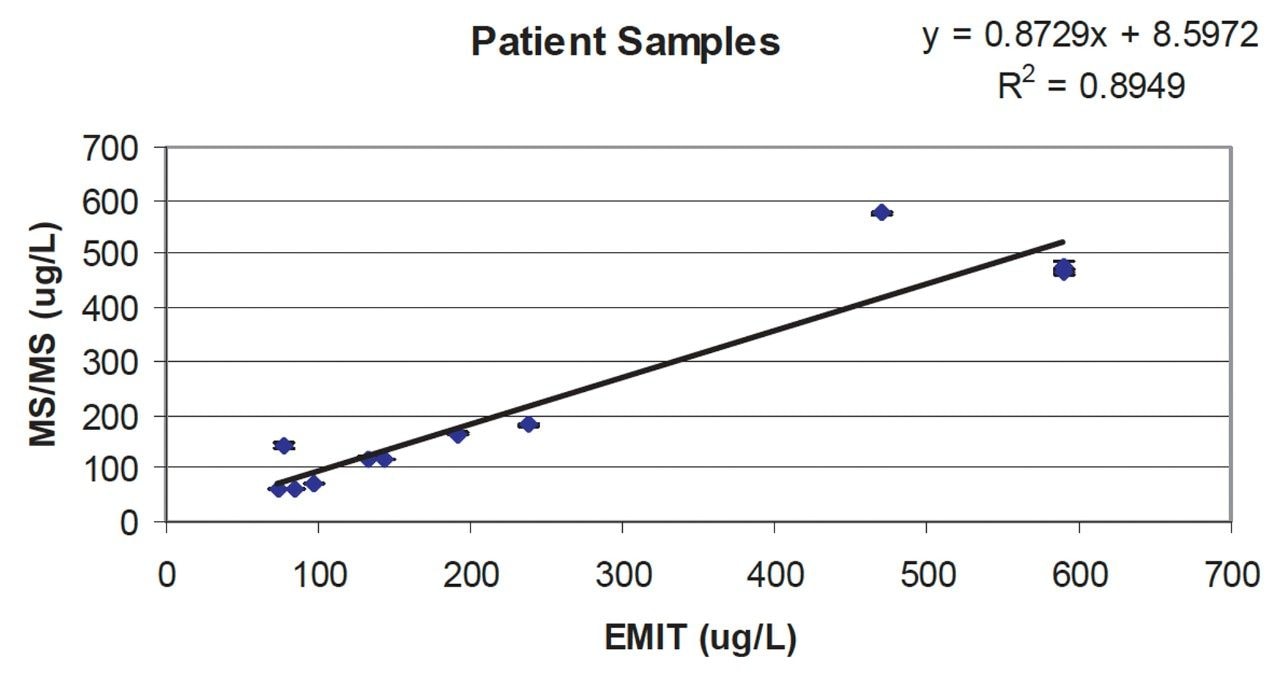
EDTA blood samples from patients on a cyclosporin régime were processed and analysed by LC-MS/MS as described. The results are compared to the values obtained by immunoassay in Figure 4. Duplicate extract analyses showed good reproducibility and a reasonable correlation with the immunoassay results (r2 = 0.895), although the origin of the uncertainties is unknown.
Tandem mass spectrometry offers a viable alternative to immunoassay for the analysis of cyclosporin in human blood samples. A simple LC-MS/MS method involving solvent precipitation and on-line sample clean-up has been described. The analytical time is approximately two minutes per sample. The method can be extended to include tacrolimus and possibly other relevant drugs. The limit of detection is below the therapeutic range of interest.
WA41862, April 2002