Accelerating Method Development and Manufacturing of GLP-1 Analogs with LC-UV/MS
Duanduan Han, Samantha Ippoliti, Robert E. Birdsall, Karen Nyholm
Waters Corporation United States
Published on May 14, 2025
Abstract
Glucagon-like-peptide-1 (GLP-1) receptor agonists have recently gained significant attention as a metabolic regulator for treating type 2 diabetes and obesity resulting in unprecedented demand. In response, manufacturers are actively reassessing their production processes to enhance efficiency and meet growing market needs. Solutions that can utilize information more efficiently to expedite decisions in a timely manner are required to ensure that manufacturers can meet regulatory standards and drug product safety while improving productivity.
In this study, we present a QC-friendly LC-UV/MS workflow designed to alleviate the workload on analytical laboratories. Access to orthogonal mass data allows supporting labs to make quicker and more informed decisions during method development, reducing errors, and increasing overall productivity.
This solution integrates the ACQUITY™ QDa™ II Mass Detector with Empower™ Chromatography Data System (CDS) as a compliance-ready, scalable software solution for instrument control, data acquisition, review, and reporting with full audit trail capabilities coupled with the Arc™ Premier System. The capabilities of this unique LC-UV/MS platform were demonstrated through a real-world use case involving the rapid detection and putative identification of a process-related impurity in a GLP-1 analog.
Benefits
- Orthogonal mass information enables detection of impurities that may be overlooked using traditional UV-based detection, increasing confidence in results.
- ACQUITY QDa II Mass Detector enhances analytical labs’ capabilities to more effectively investigate and resolve out-of-specification results.
- The integrated LC-UV/MS workflow helps manufacturers meet regulatory requirements while ensuring both product safety and operational productivity.
Introduction
Glucagon-like-peptide-1 receptor agonists (GLP-1 RAs) are widely used to treat type 2 diabetes and obesity.1 The success of the latter has resulted in an increase in product demand, which significantly strains both supply chains and manufacturers. As a result, customers are increasingly turning to compounded drugs, which are not regulated by the FDA and pose an elevated risk to consumer safety. Drug manufacturers need reliable and robust methods to perform raw material screening, in-process control, lot release, and stability monitoring to ensure drug safety and efficacy. Solutions that can utilize information more efficiently to detect anomalies and support risk-based decisions are needed to ensure that manufacturers can meet regulatory standards and drug product safety while improving productivity.
The ACQUITY QDa II Mass Detector (Figure 1) is a cost-effective compact mass detector featuring ease-of-use on/off operation and is fully integrated with the Empower CDS, offering a compliant-ready solution while enabling the acquisition of orthogonal data for increased productivity. Access to orthogonal mass data such as this allows supporting labs to make quicker and more informed decisions during method development and manufacturing of GLP-1 analogs, reducing errors and increasing overall productivity. In this study, we demonstrate the performance of the ACQUITY QDa II Mass Detector in the detection of process-related impurities and its ability to support more informative lab investigations for out-of-specification (OOS) results.

Experimental
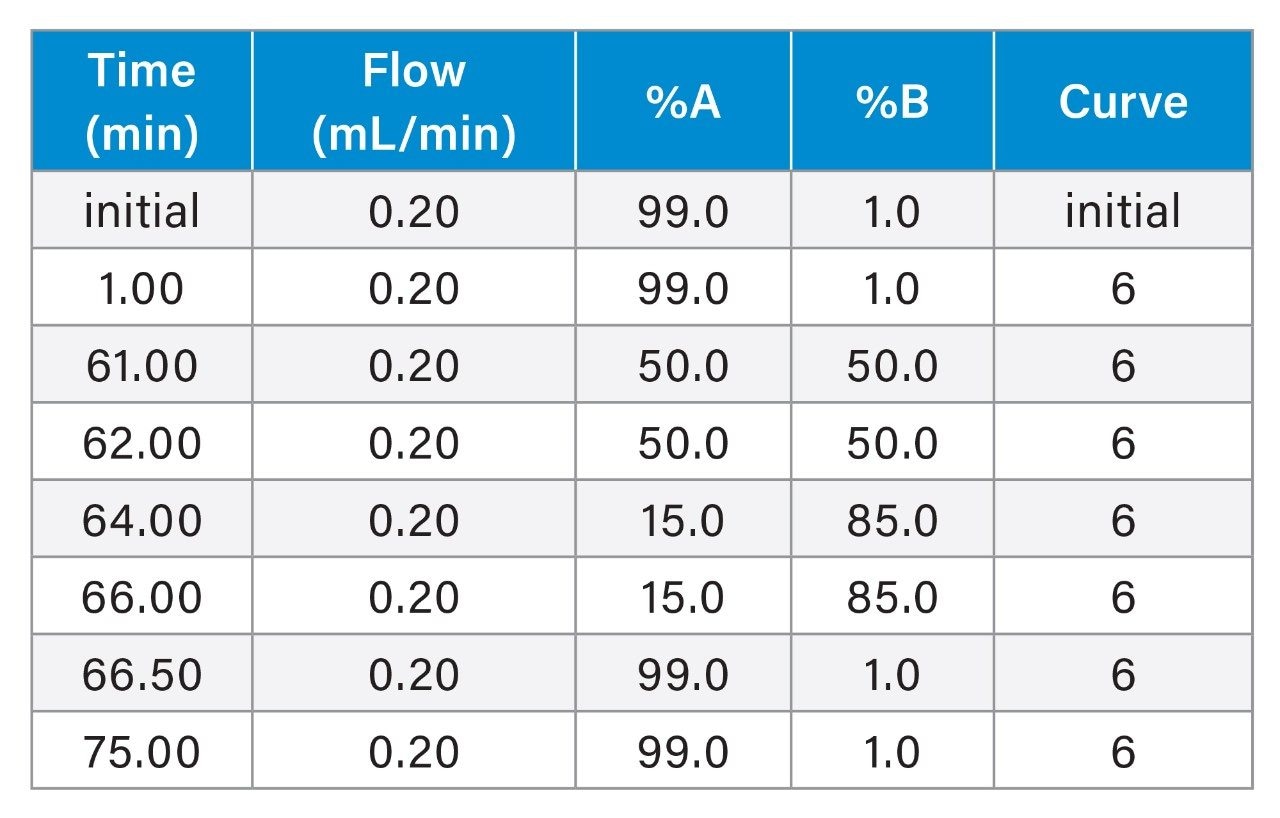
Research-grade GLP-1 analog exenatide was purchased from multiple vendors. Exenatide stock was prepared at 0.25 mg/mL using acetate buffer at pH 4.5. The stock concentration and buffer were chosen to match the formulation of corresponding drug products with minor adjustment. Exenatide was digested with RapiZyme™ Trypsin at 37 °C for 30 minutes (peptide: enzyme weight ratio 20:1). Trypsin was inactivated by adding 0.1% formic acid/water after the digestion. Thermal degradation occurred at 50 °C. Samples were analyzed at 0.1 mg/mL with the ACQUITY QDa II Mass Detector and 0.004 mg/mL with the Xevo™ G3 QTof Mass Spectrometer.
|
|
|---|---|
|
LC system: |
LC system: |
|
LC system: |
Arc Premier System (QSM) |
|
Detection: |
Detection: |
|
Detection: |
PDA, λ = 210–400 nm ACQUITY QDa II Mass Detector |
|
Column: |
Column: |
|
Column: |
XSelect™ Premier Peptide CSH™ C18 Column, 130 Å, 2.5 µm, 4.6 x 100 mm (p/n: 186009908) (+eConnect™ 186009908RF) |
|
Column temperature: |
Column temperature: |
|
Column temperature: |
60 °C |
|
Sample temperature: |
Sample temperature: |
|
Sample temperature: |
10 °C |
|
Injection volume: |
Injection volume: |
|
Injection volume: |
10 µL |
|
Flow rate: |
Flow rate: |
|
Flow rate: |
0.96 mL/min |
|
Mobile phase: |
Mobile phase: |
|
Mobile phase: |
A: 0.1% formic acid in water B: 0.1% formic acid in acetonitrile |
|
Chromatography software: |
Chromatography software: |
|
Chromatography software: |
Empower 3.8.1 |
|
|
|---|---|
|
Ionization mode: |
Ionization mode: |
|
Ionization mode: |
ESI+ |
|
Acquisition mode: |
Acquisition mode: |
|
Acquisition mode: |
Full scan |
|
Acquisition range: |
Acquisition range: |
|
Acquisition range: |
250–1500 m/z |
|
Scan rate: |
Scan rate: |
|
Scan rate: |
5 Hz |
|
Capillary voltage: |
Capillary voltage: |
|
Capillary voltage: |
1.5 kV |
|
Cone voltage: |
Cone voltage: |
|
Cone voltage: |
15 V |
|
Probe temperature: |
Probe temperature: |
|
Probe temperature: |
600 °C |
Gradient Table

|
|
|---|---|
|
LC system: |
LC system: |
|
LC system: |
ACQUITY Premier System (BSM) |
|
Detection: |
Detection: |
|
Detection: |
TUV, λ = 214 nm Xevo G3 QTof Mass Spectrometer |
|
Column: |
Column: |
|
Column: |
ACQUITY UPLC™ Premier CSH C18 130 Å, 2.1 x 100 mm, 1.7 µm (p/n: 186009488) |
|
Column temperature: |
Column temperature: |
|
Column temperature: |
60 °C |
|
Sample temperature: |
Sample temperature: |
|
Sample temperature: |
10 °C |
|
Injection volume: |
Injection volume: |
|
Injection volume: |
10 µL |
|
Flow rate: |
Flow rate: |
|
Flow rate: |
0.20 mL/min |
|
Mobile phase: |
Mobile phase: |
|
Mobile phase: |
A: 0.1% formic acid in water B: 0.1% formic acid in acetonitrile |
|
Chromatography software: |
Chromatography software: |
|
Chromatography software: |
UNIFI™ Scientific Information System |
|
|
|---|---|
|
ESI spray voltage: |
ESI spray voltage: |
|
ESI spray voltage: |
1.0 kV |
|
Sample cone: |
Sample cone: |
|
Sample cone: |
20 V |
|
Source offset: |
Source offset: |
|
Source offset: |
30 V |
|
Source temperature: |
Source temperature: |
|
Source temperature: |
120 °C |
|
Desolvation temperature: |
Desolvation temperature: |
|
Desolvation temperature: |
450 °C |
|
Cone gas: |
Cone gas: |
|
Cone gas: |
50 L/h |
|
Desolvation gas: |
Desolvation gas: |
|
Desolvation gas: |
800 L/h |
|
Collision cell RF: |
Collision cell RF: |
|
Collision cell RF: |
400 V |
|
ESI mode: |
ESI mode: |
|
ESI mode: |
Positive, sensitivity mode |
|
Scan range: |
Scan range: |
|
Scan range: |
50–2000 m/z |
|
Collision energies: |
Collision energies: |
|
Collision energies: |
Low: 6 V High: 20–50 V |
Gradient Table
Results and Discussion
Orthogonal Mass Detection
Hyphenated workflows such as LC-UV/MS offer a unique platform to help expedite the development and manufacturing process. As shown in Figure 2, the ACQUITY QDa II Mass Detector offers the simplicity of on/off operation with instrument control and settings accessed through the instrument method within the Empower CDS. This type of compact mass detector is advantageous to GLP-1 analogs and peptide-based analyses alike as mass information can quickly be ascertained to verify sample integrity and identity (Figure 2B). Hyphenated platforms such as LC-UV/MS not only increase the transferability of methods between labs but can also expedite work as access to orthogonal mass information allows support labs to make informed decisions quicker, reduce errors, and increase overall productivity.

Orthogonal Screening for Impurity Detection
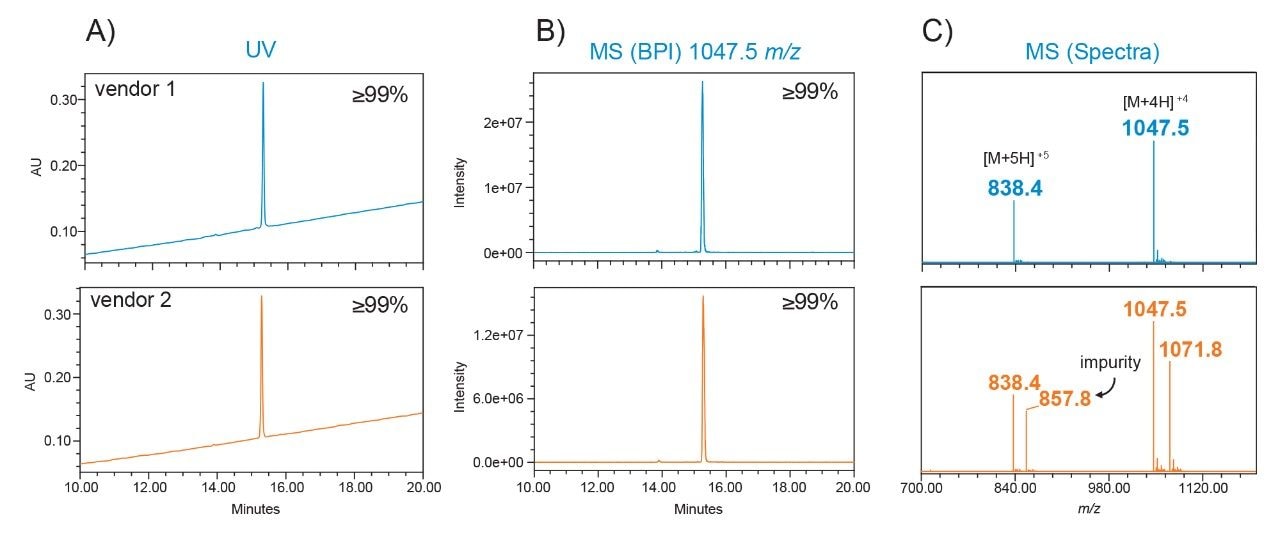
Using GLP-1 analogs as a case study, exenatide samples purchased from two different vendors were tested for comparability using this LC-UV/MS workflow. As shown in Figure 3A, UV detection indicated high purity for both samples. Similarly, MS-response for the base peak intensity (BPI) chromatogram from the ACQUITY QDa II Mass Detector also indicated high purity (Figure 3B). Interestingly, the spectral intensity for vendor 2 was observed to be half as intense (peak height 1.2e7) compared to vendor 1 despite being prepared at the same concentration. This observation prompted a closer inspection. Spectral data, as shown in Figure 3C, indicates an impurity present in vendor 2 with the presence of an additional ion series exhibiting a mass-to-charge ratio of 857.8 m/z and 1071.8 m/z. These ions, which were determined to be the [M+5H]+5 and [M+4H]+4 charged species, represent a mass difference of +97.1 Da when compared to the expected mass of exenatide. This impurity, which accounts for up to 40% of the sample based on spectral abundance, was overlooked using LC-UV alone, demonstrating the value that orthogonal mass detection provides in confirming sample identity as well as efficiently screening for impurities in manufacturing settings.
Supporting OOS Investigations
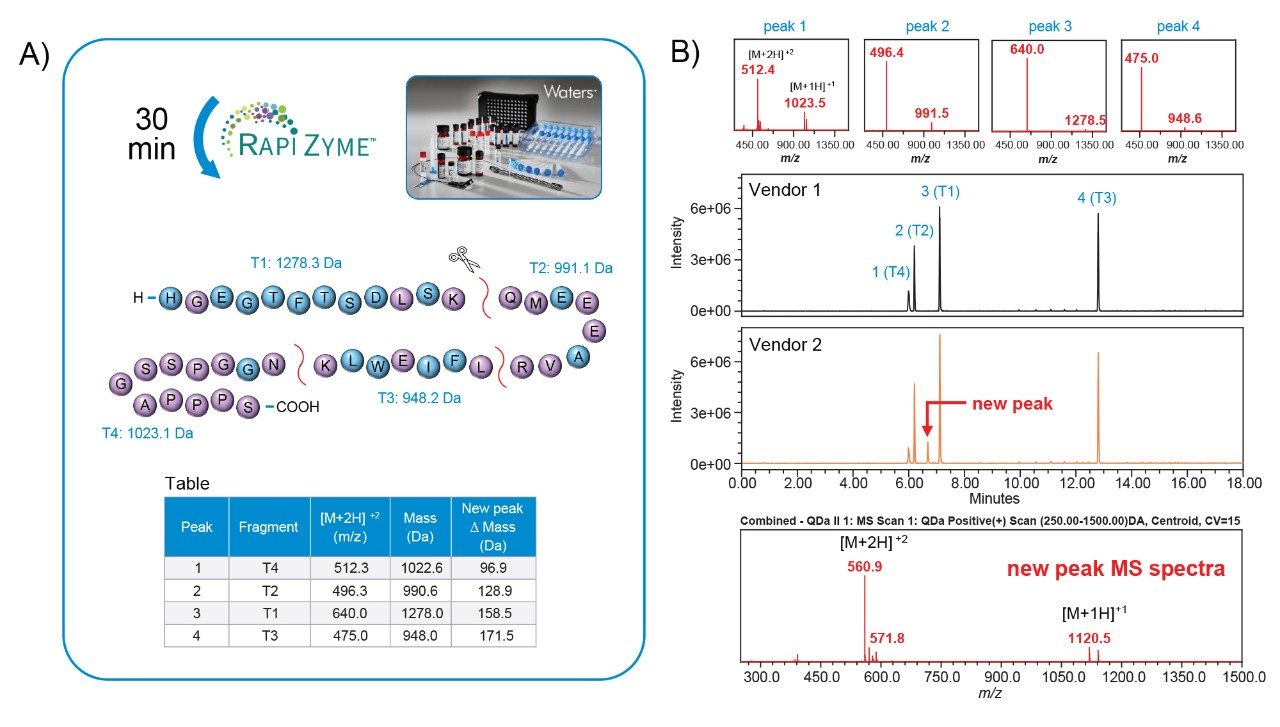
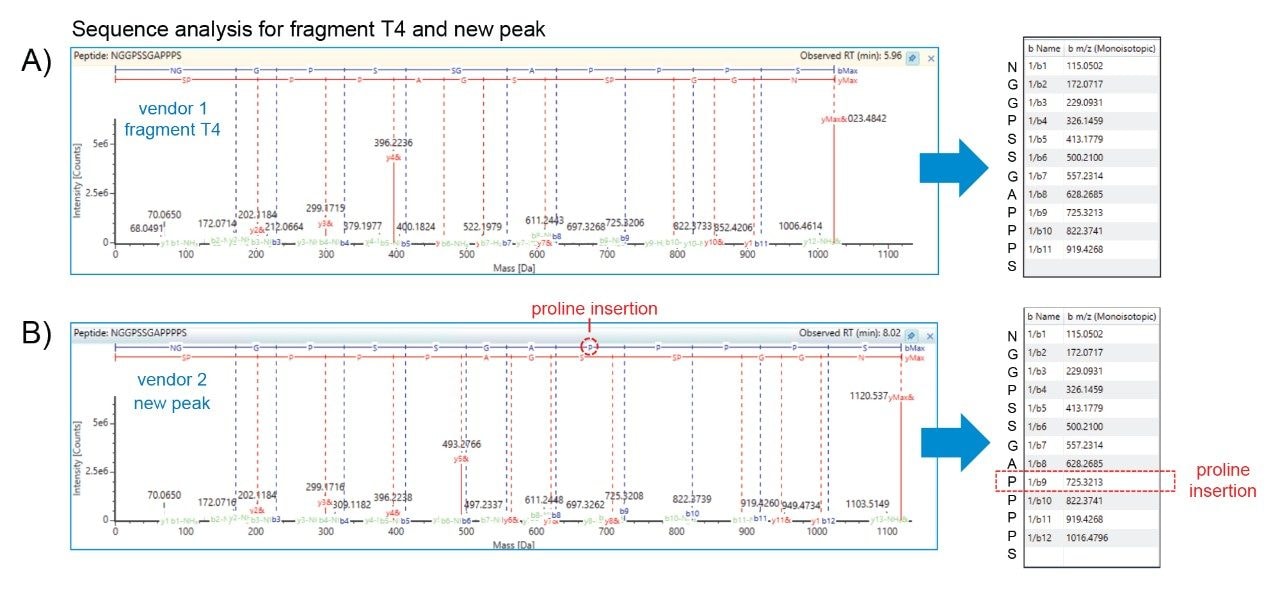
Furthermore, LC-UV/MS workflows offer the capability to support more informative analyses as part of OOS investigations. To demonstrate this, both samples were analyzed at a peptide level to gain more insights into the detected impurity. Using RapiZyme Trypsin, a quick 30-minute digestion was carried out on the samples to generate four unique peptide fragments as shown in Figure 4A. These four fragments are readily separated using reversed-phase liquid chromatography (Figure 4B) and can be identified by comparing the observed masses with expected peptide mases obtained by in silico digestion (Table, Figure 4A). Exenatide from vendor 2 generated a fifth fragment with an observed mass of 1,119.5 Da based on the ion series shown in the bottom of Figure 4B. As indicated in the table, the mass difference when compared to the four expected fragment masses could represent an insertion event of a proline, glutamine, lysine, or glutamic acid within ± 1.0 Da accuracy for peak 1 and 2. The close agreement between the observed intact mass difference (Figure 3, +97.1 Da) and peptide fragment T4 mass difference (Table, Figure 4A), putatively suggests the observed impurity is related to a proline insertion. Subsequent confirmation of the insertion event was performed using Waters Xevo G3 QTof Mass Spectrometer to perform high resolution mass analysis and sequence analysis. As shown in Figure 5, The UNIFI Scientific Information System sequencing software was able to confirm the impurity as a proline insertion occurring in the proline rich region of the C-terminus end of exenatide from vendor 2. This data demonstrates that the ACQUITY QDa II Mass Detector not only has value in providing orthogonal mass data to expedite the detection of impurities but can be utilized to provide insightful data to avoid costly mistakes such as process deviations in the manufacturing of GLP-1 analogs.
Conclusion
Integrated LC-UV/MS workflows significantly enhance the analytical capabilities of supporting laboratories by providing complementary, orthogonal mass data. This enables faster, more confident decision-making, improving overall lab productivity without compromising safety or regulatory compliance.
In this study, the ACQUITY QDa II Mass Detector, used as an in-line mass detector, successfully identified a GLP-1 impurity that would likely have gone undetected in a UV-only workflow, highlighting its critical role in impurity analysis. As a compliance-ready solution suitable for deployment in QC environments, the ACQUITY QDa II Mass Detector reduces the burden on supporting labs by streamlining workflows, enhancing impurity detection, and helping ensure drug safety and efficacy all while meeting regulatory requirements with confidence.
References
- Yu, M. et al. Battle of GLP-1 Delivery Technologies. Advanced drug delivery reviews. (2018) 130:113-130. DOI: 10.1016/j.addr.2018.07.009.
720008800, May 2025