Using the excellent reproducibility of the Waters Alliance HT HPLC System coupled to the highly sensitive and selective Micromass Quattro micro we were able to develop and validate a method for the bioanalytic study of alprazolam which met FDA guidelines for bioanalytical method validation. All data were acquired using a secure installation of MassLynx v4.0 Software with the QuanLynx Application Manager which are designed to work in a 21 CFR 11 compliant environment.
The method shows acceptable precision and accuracy throughout the calibration range which resulted in good linearity
The requirements of the regulatory bodies, such as the FDA,1 are very stringent for the bioanalytical laboratory. This makes the need for highly selective and sensitive analytical methodologies for the quantitative evaluation of drugs, very important in drug discovery development.
Obtaining low levels of detection with very selective and sensitive methodologies is the key for monitoring the therapeutic dose level in order to ascertain the efficacy of the drug. This report describes the validation of a high performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS) procedure for the measurement of alprazolam in human plasma.
Tandem MS with liquid chromatography offers an integrated solution for bioanalytical studies as it generates accurate and unambiguous data. Using MassLynx v4.0 Software, the data can be obtained in a 21 CFR part 11 compliant environment. The software maintains the integrity of the data by controlling the whole process from generation of tuning parameters (initial stages of method development) to the storage and archiving of the final validation report.
Bioanalytical method validation has a variety of important parameters, which are used for the quantitative measurements of analytes in a given biological matrix, such as plasma. These parameters are crucial for regulatory compliance on the validation of bioanalytical methods. These parameters include:
Accuracy – the closeness of the mean test results obtained by the method to the true concentration of the analyte.
Precision – the closeness of individual measures of an analyte, after multiple analysis.
Selectivity – the ability of the method to differentiate and quantify the analyte in the biological matrix, from any endogenous material.
Sensitivity – to routinely give easily determinable values at the lowest end of a calibration series.
Reproducibility – the precision of a method under same conditions over a period of time.
Stability – this is a function of storage conditions, chemical properties, the matrix and container system.

For validation purposes the accuracy and precision are determined with mean values within ±15% of theoretical values, except at the lower limit of quantification (LLOQ) where it is ±20%.
Waters Micromass Quattro micro Tandem Mass Spectrometer
Waters 2795 Separations Module
MassLynx Data Acquisition System
QuanLynx Application Manager
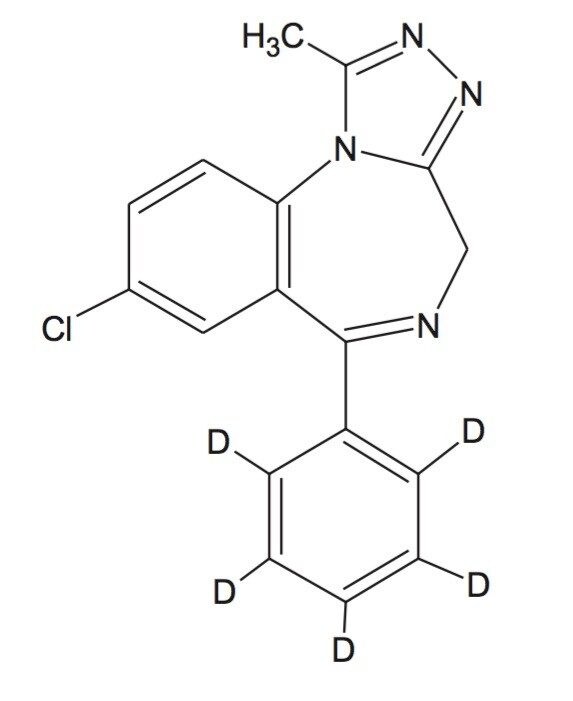
Alprazolam, alprazolam-D5 (internal Standard), acetonitrile, ammonium acetate, water, blank human plasma from Sigma-Aldrich Chemical Co. Poole, UK.
Mobile Phase
Mobile phase A – (95% 5mM ammonium acetate, pH, 5% acetonitrile) 0.04% acetic acid
Mobile phase B – (5% 5mM ammonium acetate, pH, 95% acetonitrile) 0.04% acetic acid
Flow – 400 μL/min
Column = YMC-Pack ODS-AQ (50 x 2.1 mm id)
Alprazolam primary stock solution (1 mg/mL) and working standard solutions A primary stock solution of alprazolam was prepared at a concentration of 1 mg/mL by accurately weighing out 5 mg of alprazolam into a flask and dissolving in 5 mL of mobile phase.
Internal standard primary stock solution (100 ng/μL) Internal standard solution was prepared by diluting the stock solution 1 in 10 with acetonitrile/water, 50/50, (v/v).
Alprazolam secondary stock solutions (100 ng/μL, 1 ng/μL and 100 pg/μL) The 1 mg/mL solution was diluted 1 in 10 with 50/50 (v/v), acetonitrile/water to produce a working solution. Further dilutions were made to produce the 1 ng/μL and 100 pg/μL solutions.
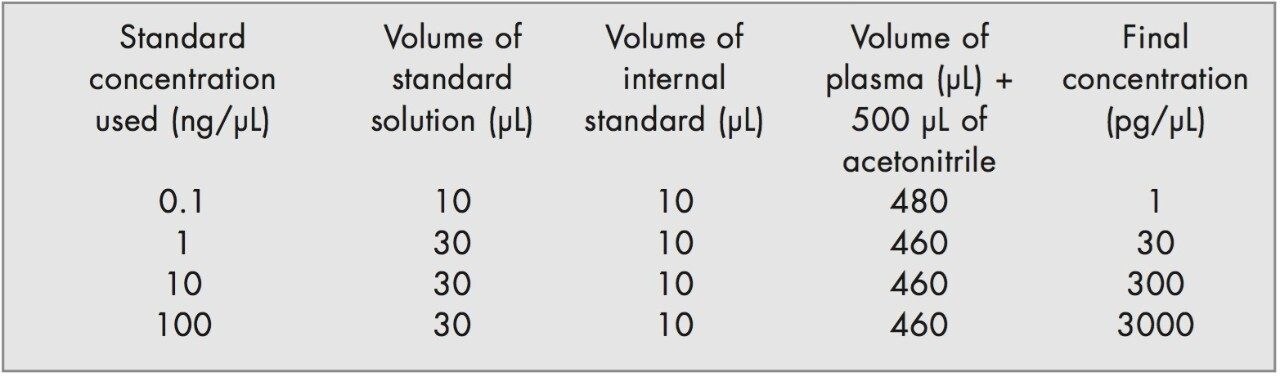
Aliquots of alprazolam working standard solutions were added to blank human plasma on the day of the analysis to produce a calibration line at the concentrations stipulated below.
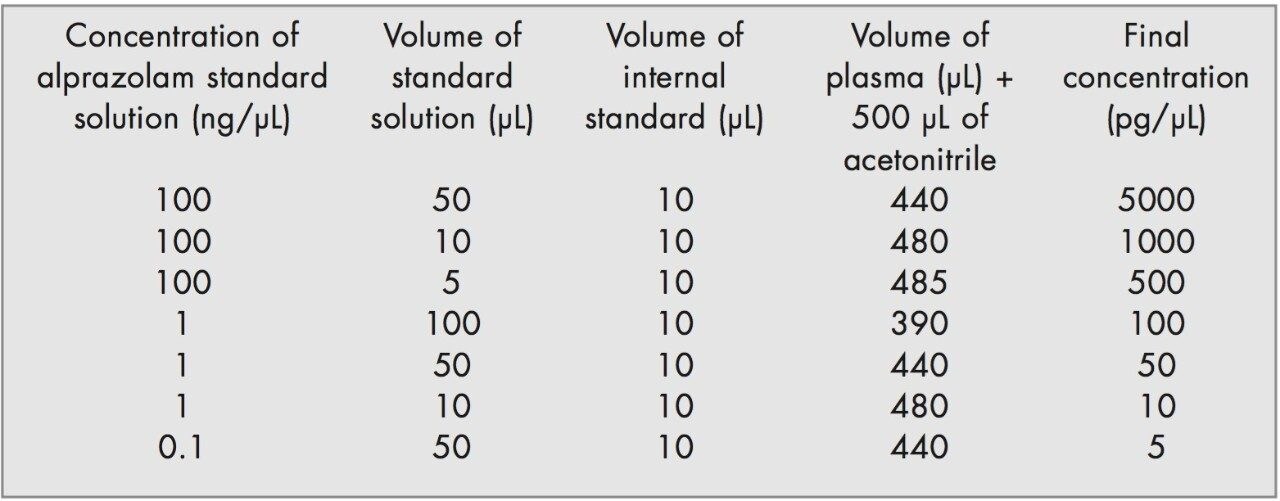
Quality control (QC) samples were prepared to assess the precision of the analytical method, the stability of the alprazolam in plasma and the recovery. The QC samples were prepared independently using stock solutions from the working standards. The samples were prepared according to the table below.
The QC samples were divided into aliquots of 25 μL and placed into sample vials. The initial prepared samples were analysed immediately and the remaining samples were stored at -22 °C prior to analysis.
A standard mixture solution was prepared containing 100 pg/μL of both alprazolam and alprazolam-D5 in acetonitrile/water (50/50, v/v).
1. The method consisted of mixing human plasma, varying amounts depending on the standard used (see table) with internal standard (alprazolam-D5 at 100 pg/μL, 10 μL).
2. The resulting mixture was vortex mixed (ca 10 seconds) and centrifuged (ca 3000 rpm, ca 3 minutes).
3. The supernatant was placed into a glass vial and injected onto a YMC-Pack ODS-AQ (50 x 21 mm id) HPLC Column.
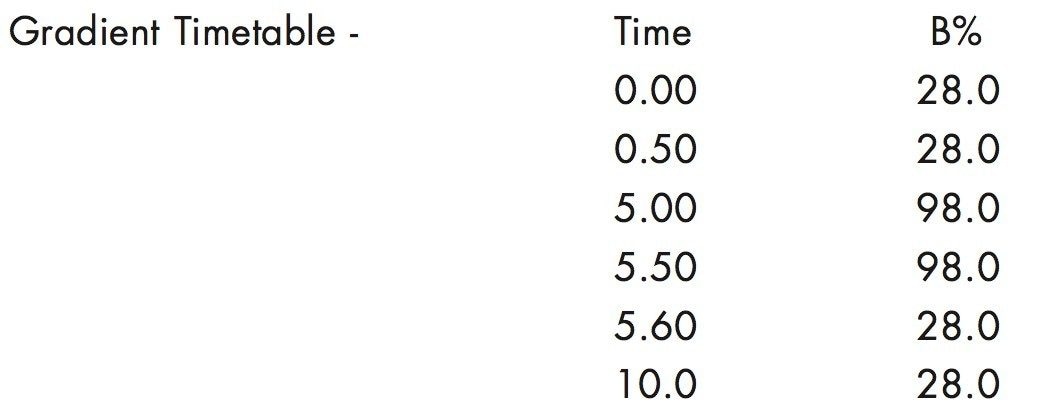
4. A 10 μL injection of the extract was run by gradient reversed-phase chromatography with a mobile phase comprising of 5 mM ammonium acetate (5%):95 acetonitrile at a flow rate of 0.4 mL/minute.
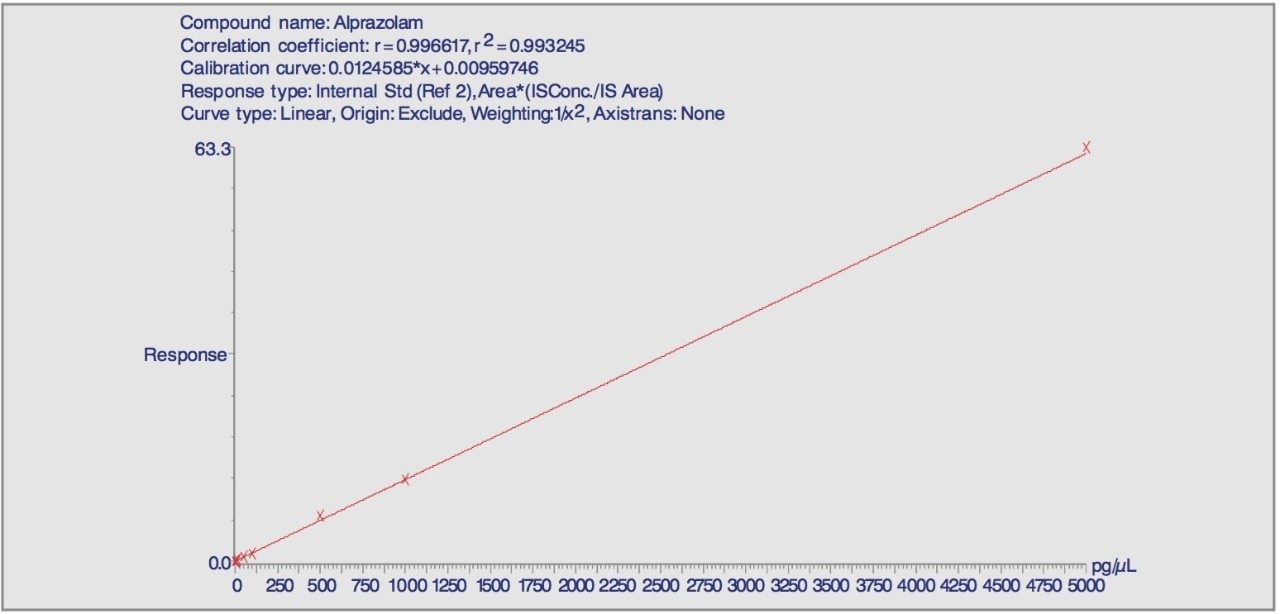
Calibration lines were prepared on the basis of peak area ratios versus concentration of alprazolam in matrix. A 1/x2 weighted least squares regression analysis of the data was used to calculate the slope, y-intercept and correlation coefficient.
Standard curves were constructed from replicate analyses of eight concentrations over the range 1 to 5000 pg/μL.
The linearity of the matrix standards was determined by plotting peak area ratios against concentration. A weighting of 1/x2 was applied in order to improve the accuracy of measurements at low levels. The information obtained included the gradient (slope), y-intercept, correlation coefficient (r), and the back-calculated calibration standard concentrations.
The precision of the assay was evaluated by the coefficient of variation (CV) within and overall from the batch analyses. A total of four different QC samples were prepared at the LOQ, low, medium and high concentrations over the calibration range. A total of eight QC samples were analyzed (two at each concentration) on a daily basis.
The accuracy was determined by measuring the QC samples and comparing them with the theoretical concentrations. The following formula was used for the calculation of the relative error (RE):
RE (%) = (Mean calculated conc. - theoretical conc.) X 100 (%)
The LOQ of the assay was the lowest concentration of the calibration series with a precision of ±20%.
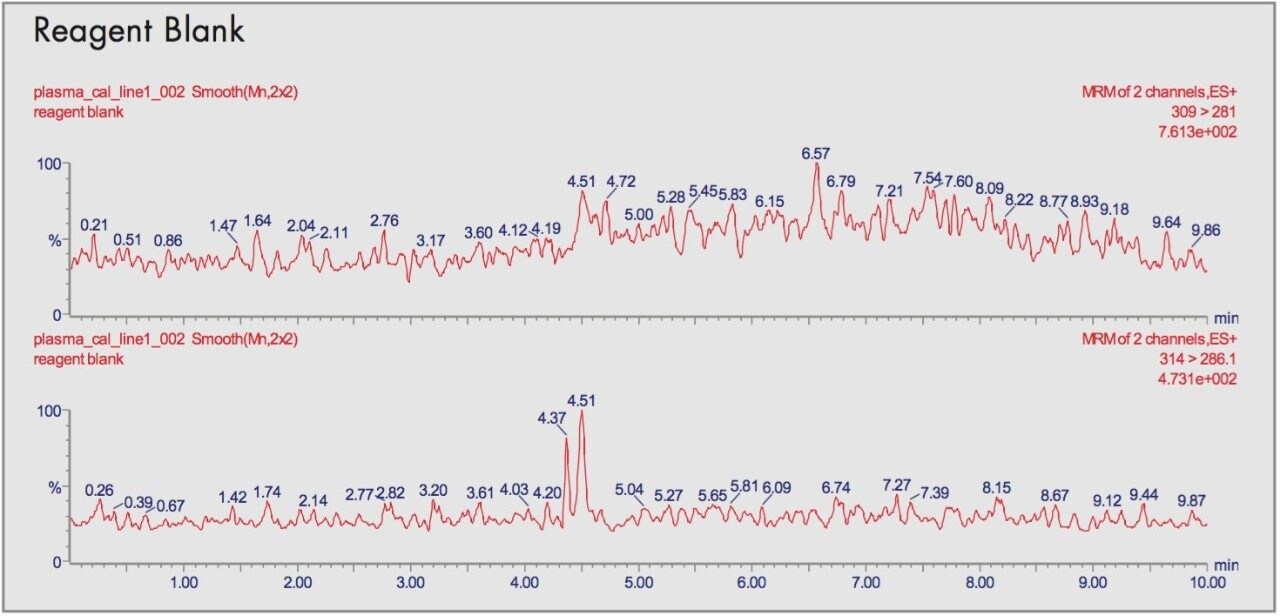
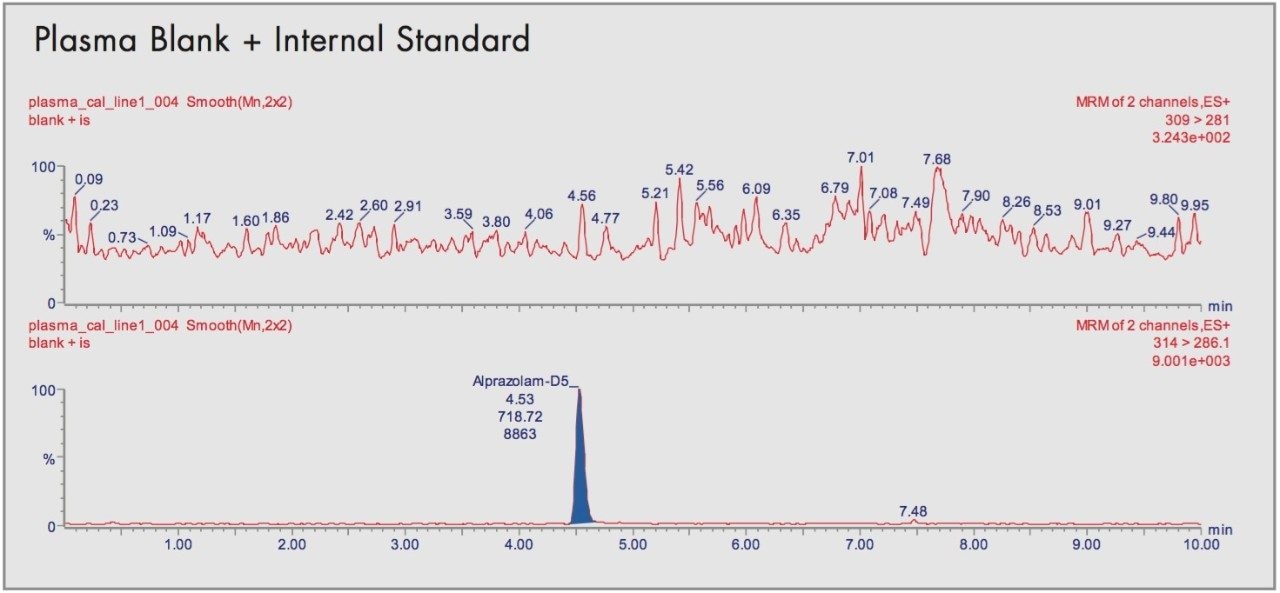
This was ascertained by comparing the blank human plasma with the spiked plasma in order to see any interfering peaks at the same retention times and mass transition.
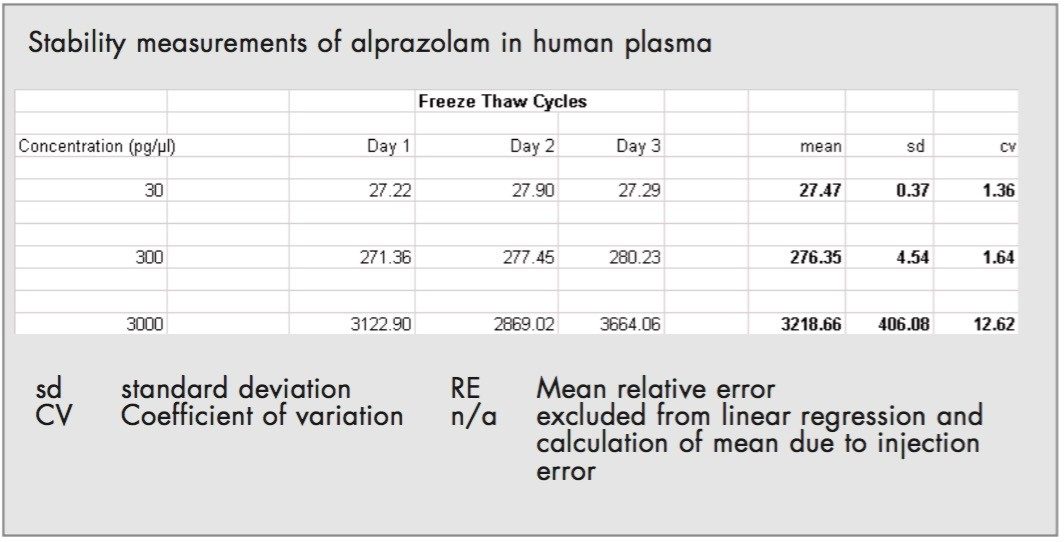
This was monitored by the freeze thaw cycles. As the first QC samples were prepared they were analyzed immediately. Then the remaining samples were divide up into QC samples and freeze thaw QC samples. The freeze thaw QC samples were taken out of a freezer (at -22 °C) and left on the bench for 2 hours (sufficient time to defrost). They were then analyzed and returned to the freezer. This was done on day one, two and three of the validation procedure.
The standard mixture was injected prior to the calibration series and the peak areas, ratios and shape were monitored. Action such as changing mobile phase or column was taken if any significant change was observed.
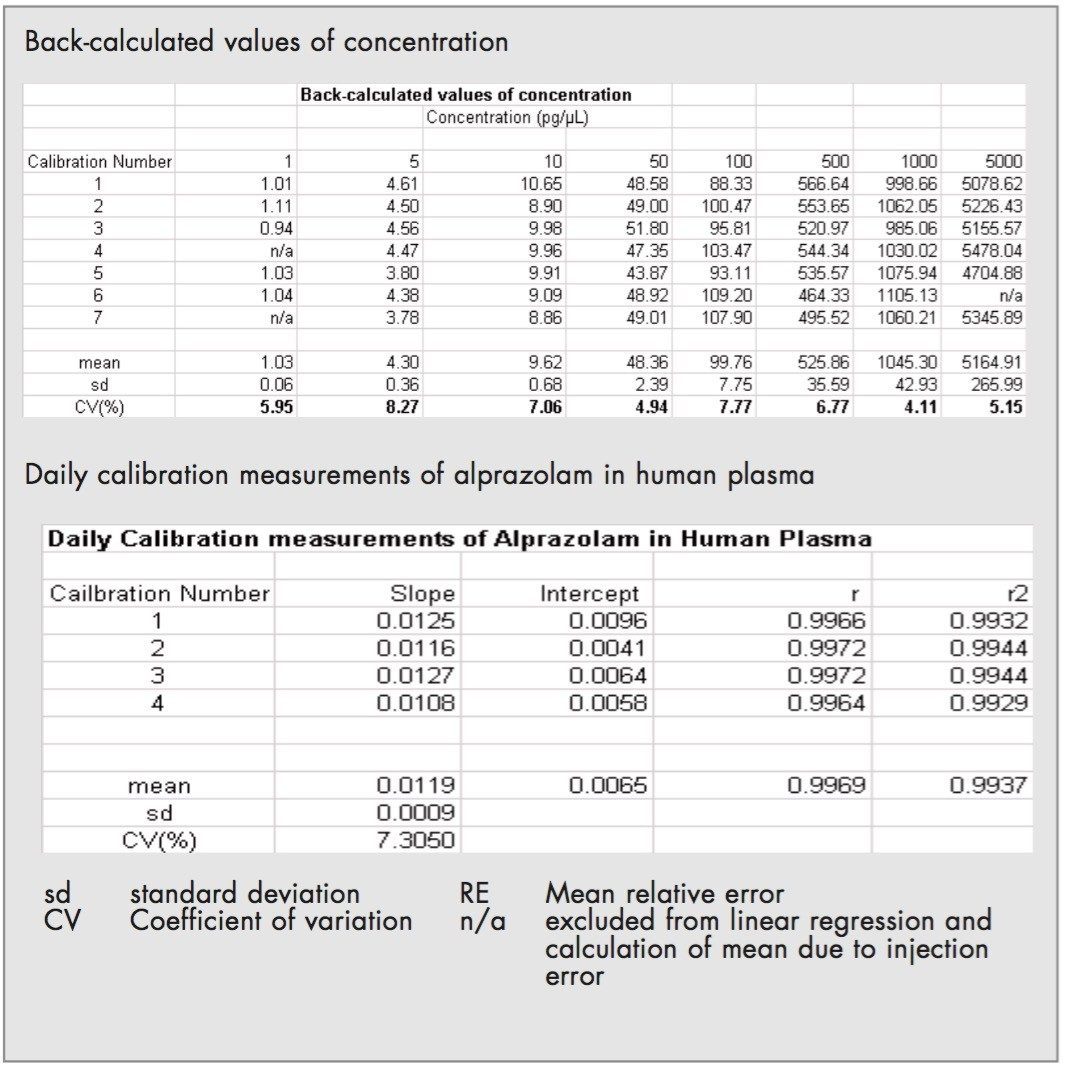
The relationship of the peak area ratio of alprazolam and the alprazolam-D5 internal standard with concentration was linear (mean r=0.9968, n=8) over the calibration range 1 to 5000 pg/μL. The back-calculated concentrations for each calibration point expressed as relative error were between -9.0% and 2.7% over the calibration range. Examples of the calibration line are shown in Figure 3 and typical chromatograms for each calibration point are shown in Figures 4A-4D.
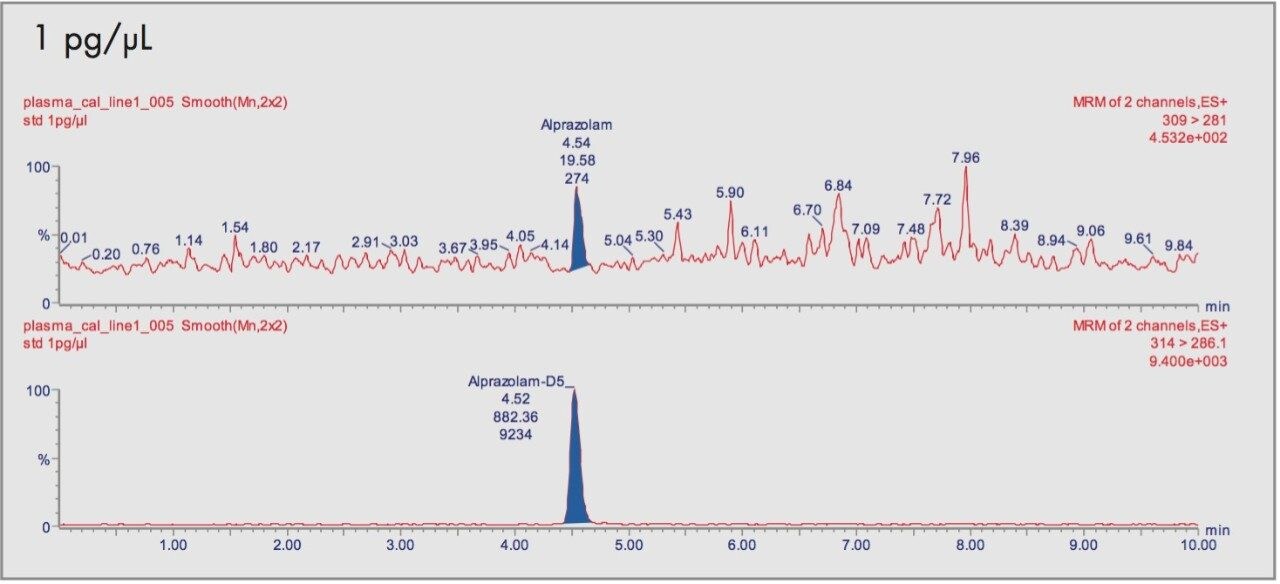
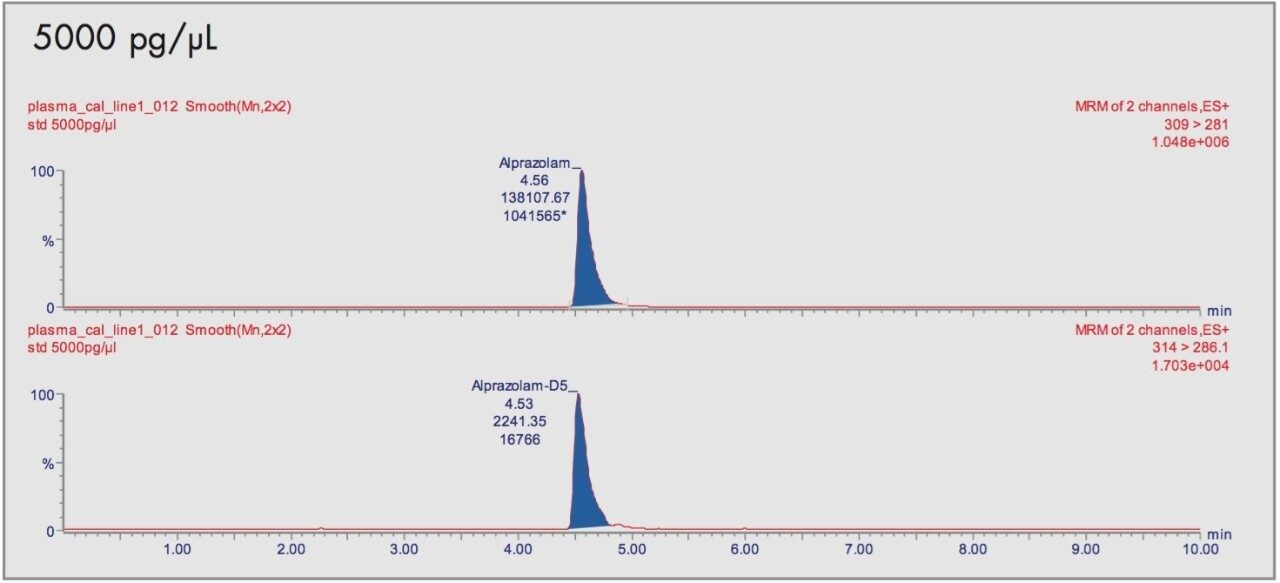
The daily calibration measurements are summarized in Table 1.
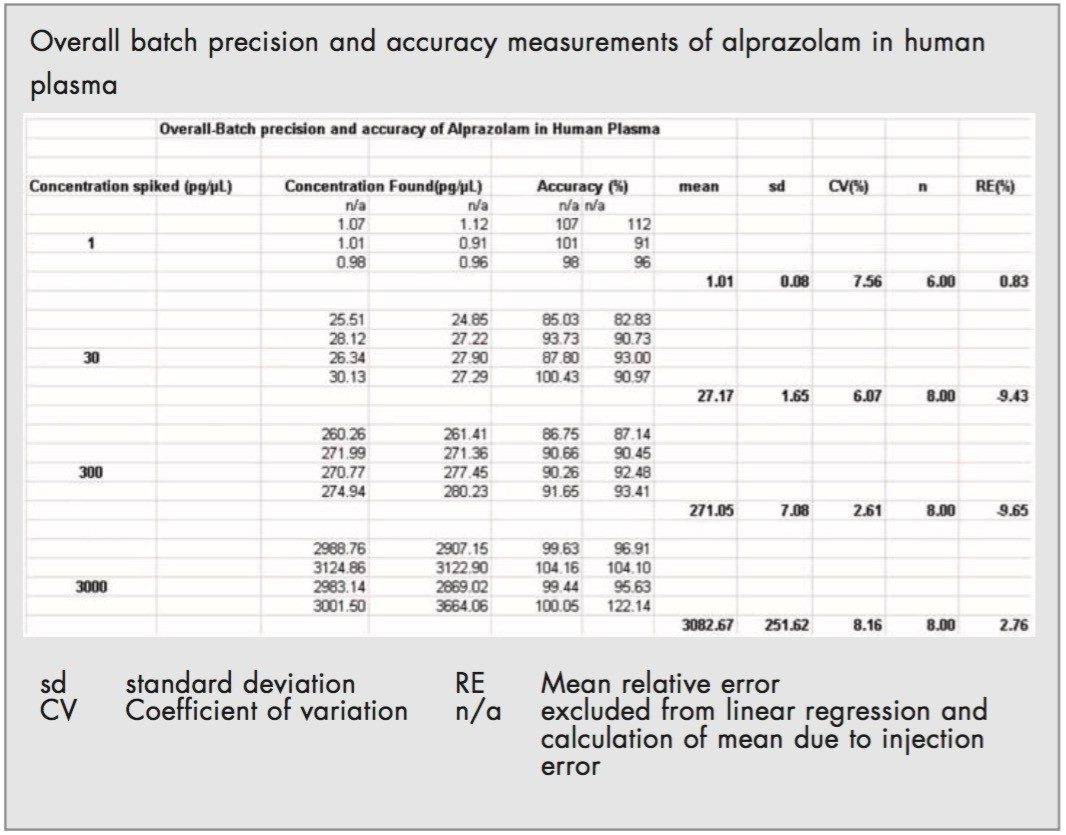
Overall batch precision measurements were 7.5% at 1 pg/μL, 6.1% at 30 pg/μL, 2.6% at 300 pg/μL and 8.2% at 3000 pg/μL (Table 2).
The already defined limit of quantification (LOQ), was 1 pg/μL in plasma using the lowest calibration standard. At this level the back-calculated concentrations gave a mean value of 1.03 pg/μL with a CV of 5.9%.
In extracts of blank human plasma there were no interfering peaks at the same retention times as the alprazolam and the internal standard. Typical chromatograms are shown in Figure 5 for the blanks and comparisons with the calibrants show no carry-over between injections.
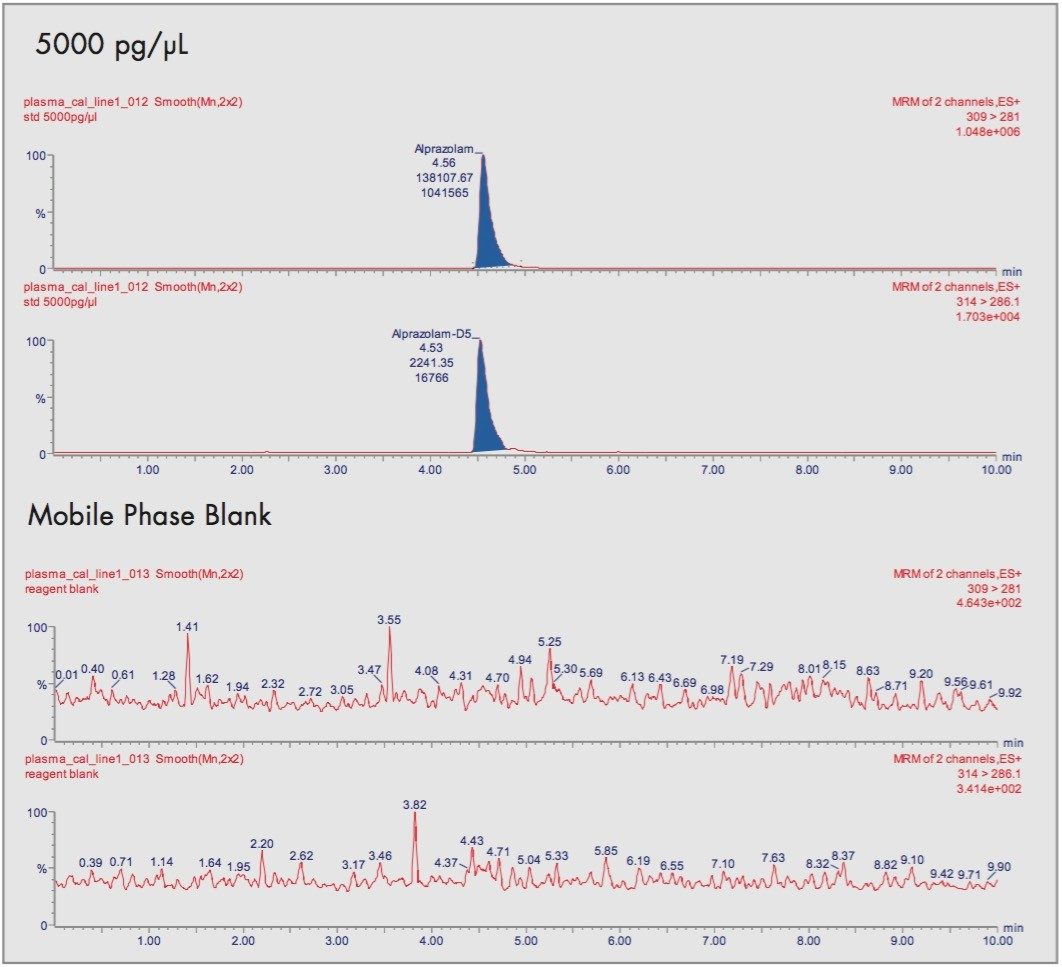
Alprazolam was shown to be stable in protein-precipitated human plasma at ambient room temperature (nominally +22 °C) for up to 2 hours, stored frozen at nominally -22 °C and following 3 freeze/thaw cycles (Table 3).
The results showed that over a calibration range of 1 to 5000 pg//L the linearity was in excess of 0.99, (Figure 3) with all standards within the range of -9 to 2.7% relative error. This is well within the accepted criteria of ±15% (20% at the lower limit of quantitation) as defined by the FDA.
The overall batch precision and accuracy for the QC's at 1, 30, 300 and 3000 pg/μL were in the range of 6.1 to 8.2%, again well within the ±15% defined. The defined limit of quantification was maintained at 1 pg/μL with a mean back-calculated concentration of 5.9%. This highlights a very precise and accurate developed method that maintained the integrity of the standards throughout the validation.
The freeze thaw cycles for the QC's at 30,300 and 3000 pg/μL were within the range 1.36 to 12.62% (coefficient of variation).
Overall the data showed excellent results from the initial method development to the final validated data. All the extracted standards and QC's (and freeze thaw QC's) gave excellent individual results well within the strict criteria set by the FDA of ±15%. The overall batch precision and accuracy also fit well within the regulatory guidlines.
It is concluded that the bioanalytical method for the measurement of alprazolam in protein-precipitated human plasma has been successfully validated over the calibration range 1 to 5000 pg/μL. The method shows acceptable precision and accuracy throughout the calibration range which resulted in good linearity. The limit of quantification was 1 pg/μL with an injection volume of 10 μL.
Alprazolam was stable in protein precipitated human plasma at room temperature for up to 2 hours, stored frozen at nominally -22 °C and following 3 freeze/thaw cycles.
Using the excellent reproducibility of the Waters Alliance HT HPLC System coupled to the highly sensitive and selective Micromass Quattro micro we were able to develop and validate a method for the bioanalytic study of alprazolam which met FDA guidelines for bioanalytical method validation. All data were acquired using a secure installation of MassLynx v4.0 Software with the QuanLynx Application Manager which are designed to work in a 21 CFR 11 compliant environment.
720000521, September 2003