LC-MS Analysis of Underivatized Bisphosphonate Drugs Using Mixed-Mode Reversed-Phase/Anion-Exchange Chromatography
Melissa L. Aiello, Kenneth D. Berthelette, Christopher Collins, Thomas H. Walter
Waters Corporation, United States
Published on September 12, 2025
Abstract
Bisphosphonates (BPs) are an important class of drugs used for the treatment of bone density loss from natural aging and from human disease. Many of the methods previously reported for these polar, acidic drugs require derivatization for analysis by LC-MS (liquid chromatography-mass spectrometry). In this application note, a 6-minute reversed-phase LC-MS method was developed for the detection of four bisphosphonate drugs without requiring a derivatization step.
For this work, a mixed-mode reversed-phase/anion-exchange column was employed, specifically the Atlantis™ Premier BEH™ C18 AX Column. The analytical data demonstrated good retention and resolution of four bisphosphonate drugs. Peak analytical data was reproducible across 6 replicate injections. Additionally, the column’s MaxPeak™ High-Performance Surfaces (HPS) hardware provided higher peak areas when compared to a stainless-steel counterpart by mitigating non-specific adsorption of the analytes to the column hardware.
Benefits
- A fast mixed-mode LC-MS method is reported for bisphosphonates that doesn’t require sample derivatization
- The Atlantis Premier BEH C18 AX Column resolved most bisphosphonates with good retention and peak shape
- The Atlantis Premier Column with HPS demonstrated higher peak areas and improved area precision compared to a conventional stainless-steel column
Introduction
BPs have played a major role in the mitigation of bone density loss caused by diseases such as osteoporosis, Paget’s Disease of bone, and bone complications related to malignancy.1 Specifically, BPs adsorb to bone surfaces and, once ingested and metabolized by osteoclastic (bone breaking) cells, inhibit their activity by blocking several key enzymatic pathways. Although patents for some bisphosphonates have ended or are about to end, their development as generic drugs is predicted to continue. Additionally, newer generation BPs called designer bisphosphonates are being synthesized with defined properties in terms of mineral affinity and actions on relevant enzymatic pathways.1 Moreover, exciting new applications in medicine such as the treatment of inflammatory bone loss, orthopedic implant fixation, and anti-parasitic effects are being investigated.
BPs are a challenge to analyze by liquid chromatography. They are highly polar acidic molecules that are not well retained on reversed-phased columns. Most of them are not readily detectable using UV absorbance. Additionally, BPs can react with metal ions to form adducts and multiple-charged substances that are difficult to detect by mass spectrometry.2 As a result, most HPLC methods for these drugs require a sample derivatization step to improve their retention and detection. This is especially needed for the analysis of BPs in complex biological matrices to help improve method sensitivity.2
In this work, a mixed-mode reversed-phase /anion-exchange (RP/AX) LC-MS method was developed to retain and separate bisphosphonate drugs using an Atlantis Premier BEH C18 AX Column. This column combines the functionality of a C18 stationary phase with anion exchange (AX) capability to retain highly polar acidic compounds. This column also employs an HPS to prevent undesired interaction with the column hardware, increasing sensitivity.
Experimental
Sample Description
Four bisphosphonate drugs were prepared as individual stock standards at a concentration of 1 mg/mL in 90:10 (v/v) water and acetonitrile. They were then combined to create a bisphosphonate standard mix. All standards were stored at 4 ºC in polypropylene containers and were aliquoted into Waters™ QuanRecovery™ Vials for analysis by LC-MS.
Method Conditions
LC Conditions
|
LC system: |
ACQUITY™ UPLC™ H-Class Plus System with HPSa tubing |
|
Mobile phase A: |
Water |
|
Mobile phase C: |
Methanol |
|
Mobile phase D: |
200 mM Ammonium Formate, pH 3 |
|
Washes/diluent: |
90:10 (v/v) Water and Acetonitrile |
|
Gradient: |
See below |
|
Flow rate: |
0.4 mL/min |
|
Injection volume: |
2 µL |
|
Columns: |
Atlantis Premier BEH C18 AX 1.7 µm, 2.1 x 50 mm (p/n: 186009407) Atlantis BEH C18 AX 1.7 µm, 2.1 x 50 mm stainless steel hardware (packed in-house) |
|
Column temperature: |
40 °C |
|
Cone, capillary voltage: |
15 V, 0.8 kV |
|
Scan mode: |
ESI negative, SIR |
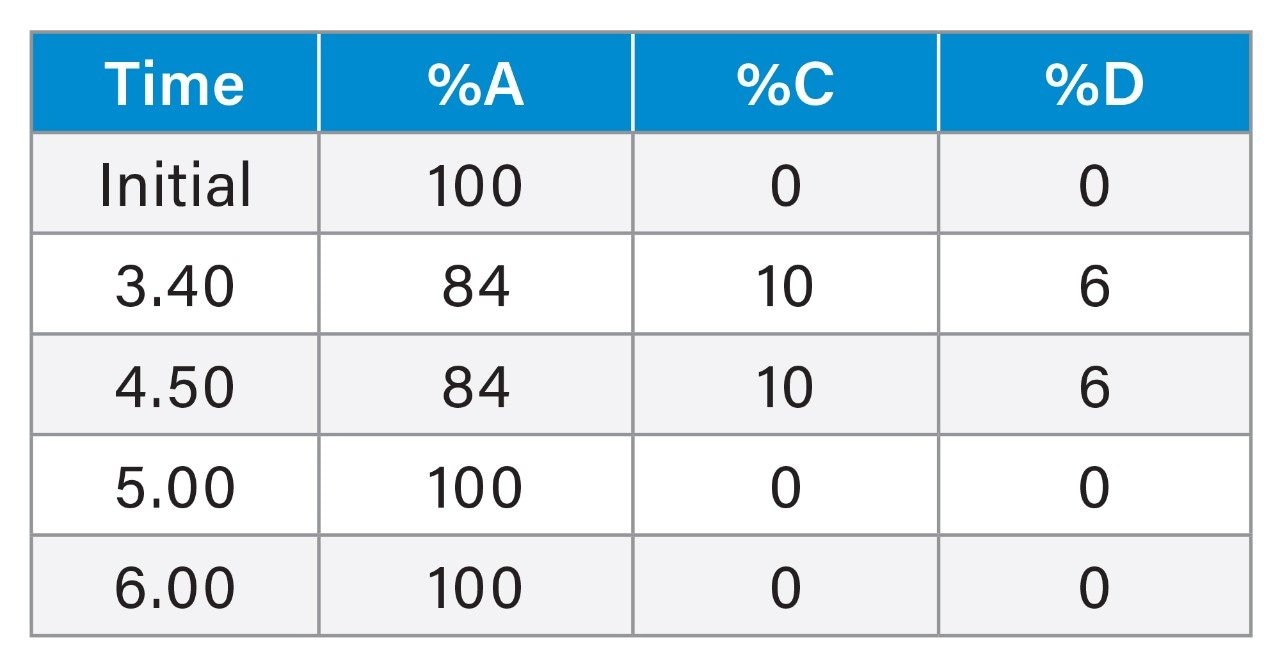
Gradient Table
MS Conditions
|
MS system: |
ACQUITY QDa™ Detector |
|
Detection mode: |
ESI- |
|
Capillary voltage: |
0.8 kV |
|
Cone voltage: |
15 V |
|
Scan mode: |
ESI negative, SIR |
Data Management
|
Chromatography software: |
Empower™ Chromatography Data System (CDS) |
Results and Discussion
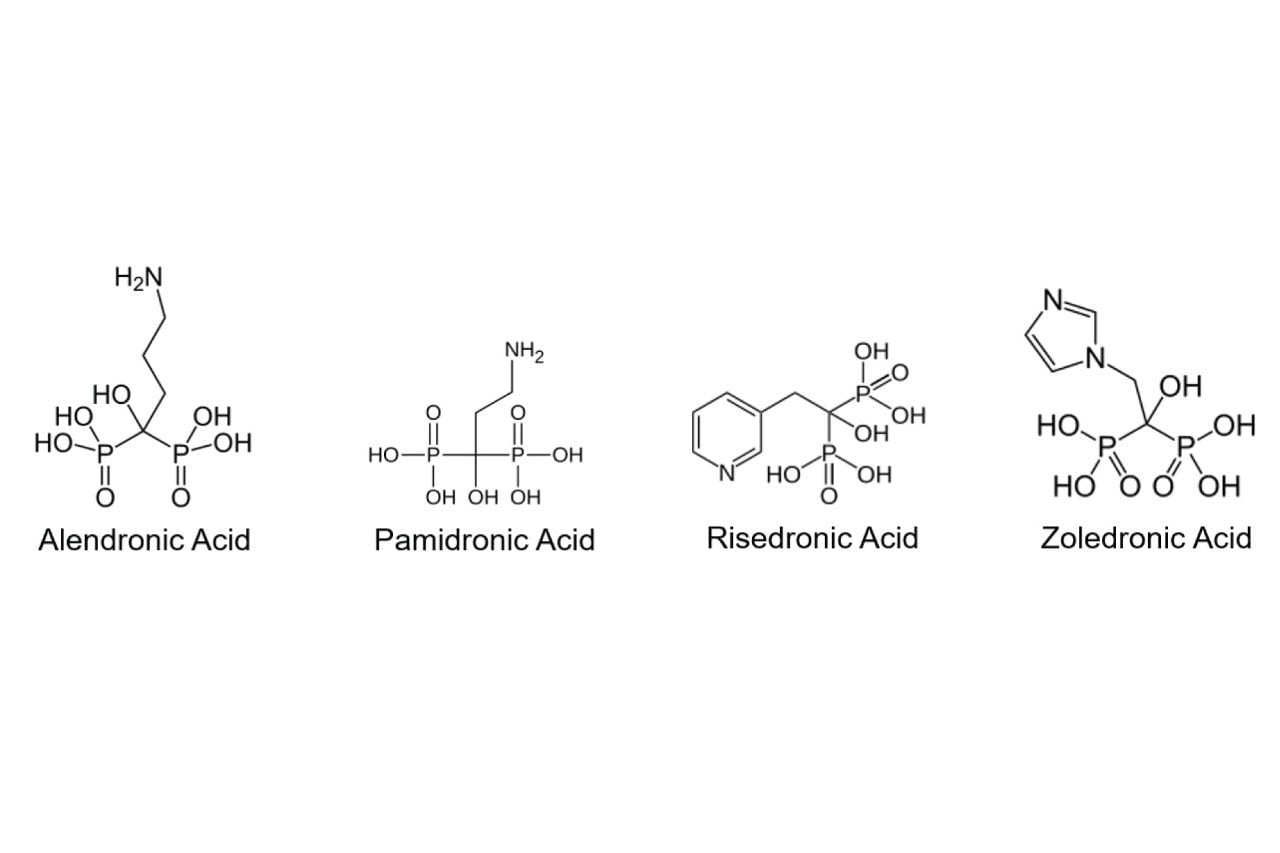
Considering the structures of the analytes (Figure 1) and the results of preliminary column screening, it was determined that a mixed-mode RP/AX column provides the best retention. These molecules are ionized above about pH 2, as the negative log of their first and second acid dissociation constants (pKa1, pKa2) are generally 2 or lower.3 While the amine substituents present in most of these drugs are protonated under acidic conditions, the presence of two phosphonate groups means that they mostly exist as anions. Consequently, they benefit from a column with anion-exchange functionality, such as Waters Atlantis Premier BEH C18 AX Column. The stationary phase in this column is bonded with both C18 and tertiary alkylamine groups, creating a positive surface charge below pH 8.4 The BEH particles used for this stationary phase have a smaller pore size than other BEH stationary phases (95 Å compared to 130 Å), providing increased retention due to a 50% higher surface area.4 Additionally, this column is usable over a wide pH range (2–10), so a variety of buffers at various pH values can be tested for method optimization.
The MaxPeak Premier Technology used in Atlantis Premier BEH C18 AX Columns was specifically designed to mitigate unwanted interactions of analytes with the column hardware. These interactions can have a detrimental impact on separations, affecting peak shape and peak areas and causing high variability.6 This is especially true for acidic analytes that can interact with the oxide layer of the stainless-steel hardware.7 In LC-MS, an additional issue is that some analytes can form adducts with metal ions released from the hardware, decreasing the intensity of the target ions. It has been demonstrated in the literature that the analytical recovery and thus sensitivity for certain metal-sensitive analytes such as citric acid cycle intermediates can be improved with MaxPeak Premier Technology.8
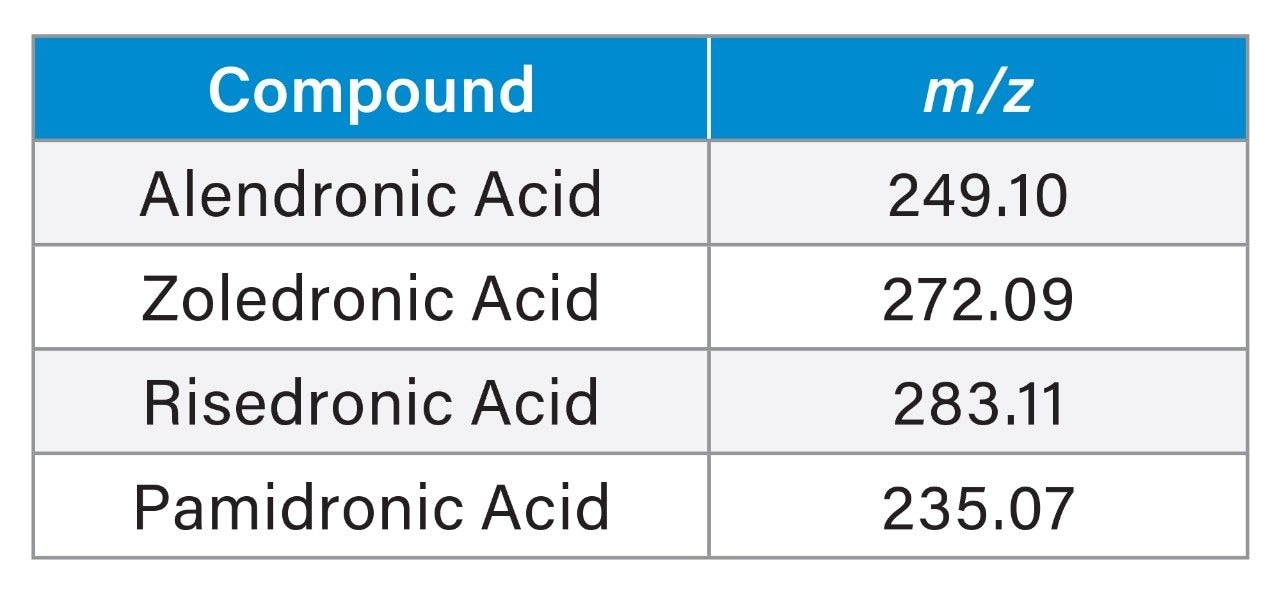
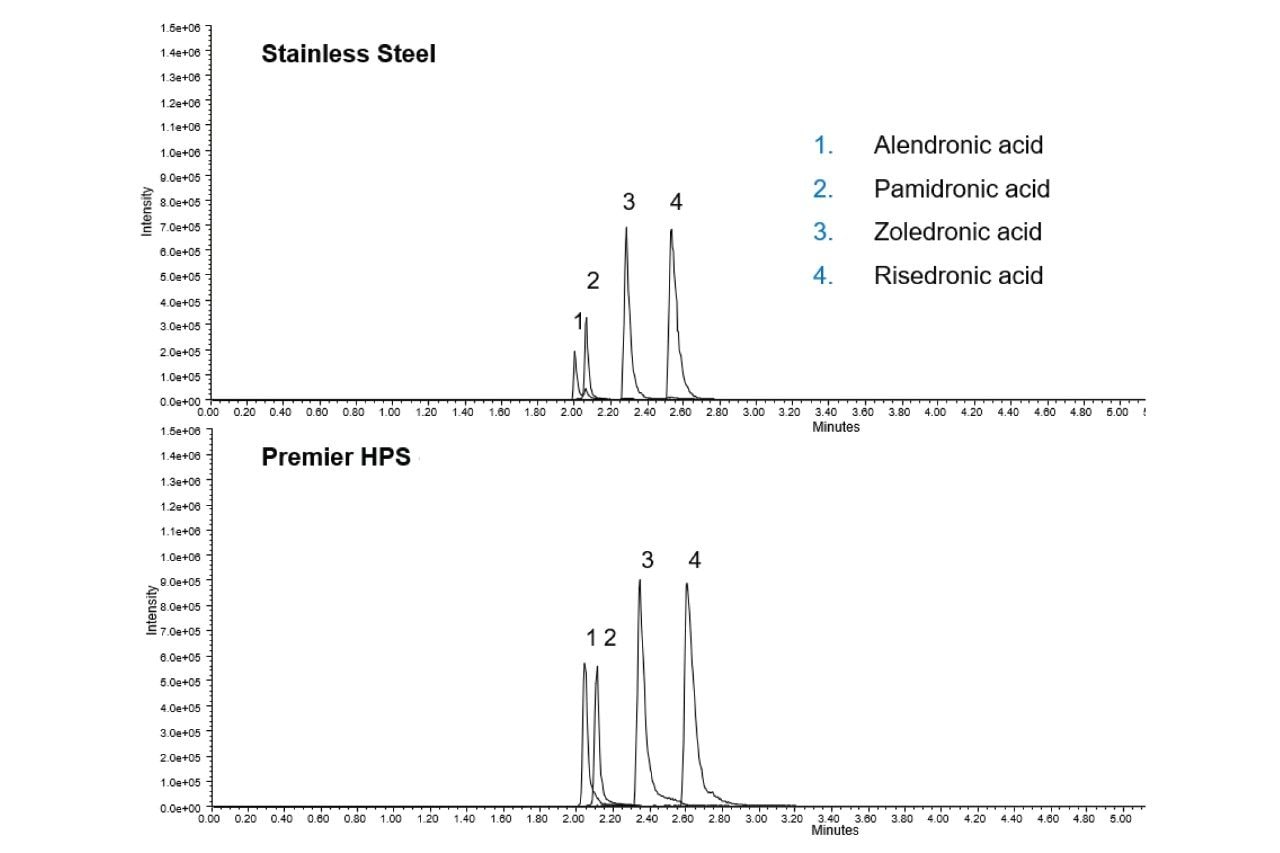
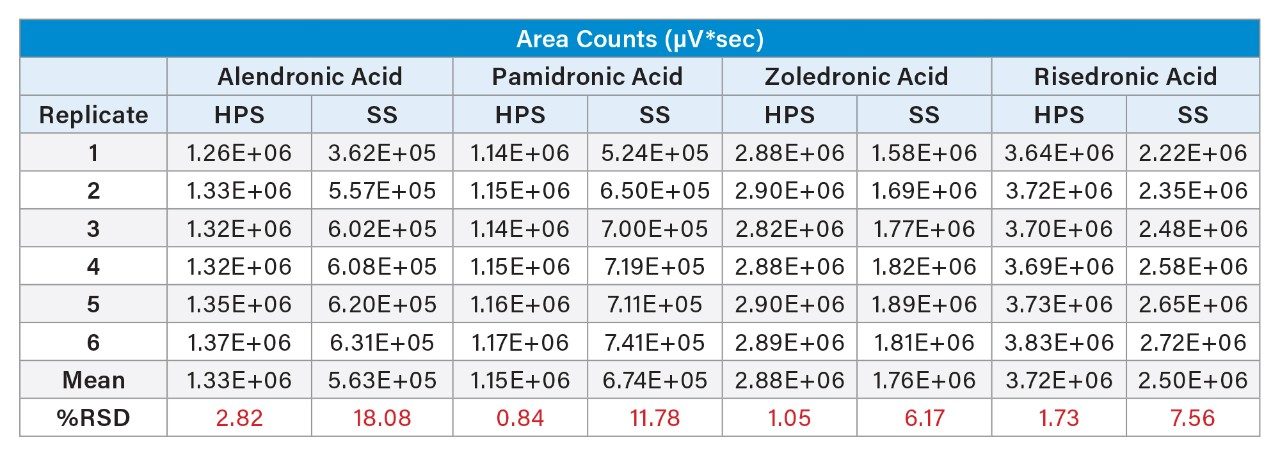
The ability of MaxPeak Premier Technology to mitigate non-specific adsorption of the bisphosphonates was evaluated. Six replicate injections of the bisphosphonate standard mix were made on an Atlantis Premier BEH C18 AX Column and were compared to the chromatography of 6 injections on a stainless-steel column packed with the same stationary phase (Figure 2). The Atlantis Premier Column showed much higher area counts. For zoledronic acid, for example (component 3), the area count for the first injection on the Atlantis Premier Column is around double the area for the stainless-steel column. Additionally, as shown in Figure 3 and Table 2, the Atlantis Premier Columns gave consistently higher area counts for all four bisphosphonates. It is interesting to note that the plots show low area counts in the early injections on the stainless-steel column that slowly rise over subsequent injections. This demonstrates the lengthy sample conditioning effect that is not necessary when using the Atlantis Premier Column.
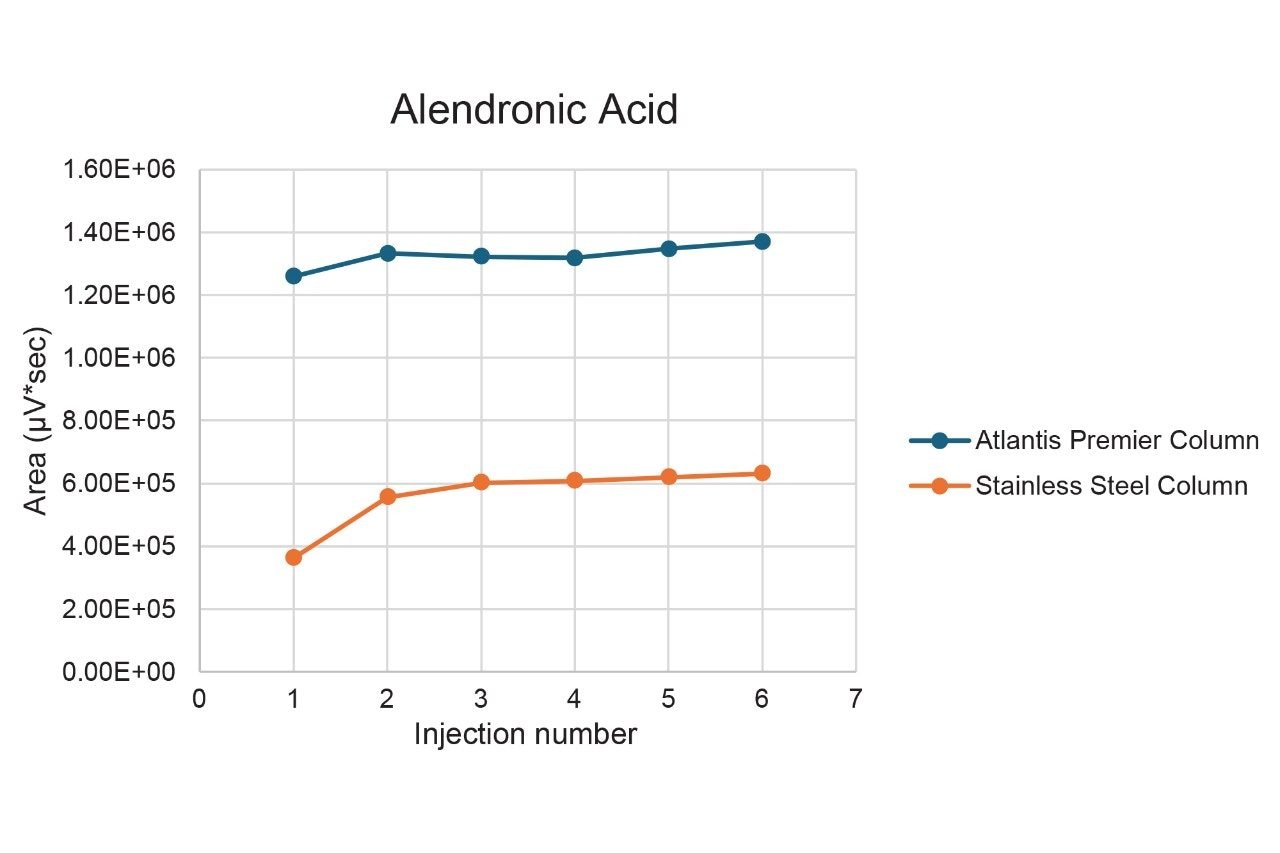
Lastly, there is also a marked difference in area count precision, with lower %RSDs for the column with the high-performance surface (Table 2). This provides evidence for decreased interactions of the analytes with the column hardware and removes uncertainty in the data.
Conclusion
Bisphosphonates can be challenging to analyze chromatographically due to their polar acidic nature. Many of the analytical methods for these drugs reported in the literature require a sample derivatization step. Mixed-mode reversed-phase/anion-exchange HPLC provides an approach for retaining and separating these analytes without derivatization. It also eliminates the need for ion-pairing agents and is compatible with mass spectrometry detection.
In this work, the development of a fast mixed-mode RP/AX LC-MS method for the analysis of four common bisphosphonate drugs is described that provides good retention and separation. The MaxPeak HPS Technology used in Atlantis Premier BEH C18 AX Columns was shown to successfully mitigate non-specific adsorption of the bisphosphonates on the column hardware, providing higher analyte peak areas and improved peak area precision compared to a stainless-steel version. This eliminates the need for lengthy column conditioning steps such as the use of chelators in the mobile phase, ion pairing agents, or sacrificial analyte injections.
References
- Graham R, Russell, G. Bisphosphonates: The first 40 years. Bone (2011) Jul;49(1):2–19.
- Chen M, Liu K, Zhong D, Chen X. Trimethylsilyldiazomethane Derivatization Coupled with Solid-Phase Extraction for the Determination of Alendronate in Human Plasma by LC-MS/MS. Anal. Bioanal. Chem. (2012) 402(2):791–798.
- Popov K, Oshchepkov M, Tkachenko S, Sergienko V, Oshchepkov A. Bisphosphonates: Synthesis, Structures, Properties, Medical and Industrial Applications. J. Mol. Liq. (2022) 351: Article 118619.
- Walter TH, Alden BA, Field JA, Lawrence NL, Osterman DL, Patel AV, DeLoffi MA. Characterization of a highly stable mixed-mode reversed-phase/weak anion-exchange stationary phase based on hybrid organic/inorganic particles. J Sep Sci. (2021), 44(5): 1005–1014.
- Meng X, Zhenwei W. A mixed-mode reversed-phase/strong-anion-exchange stationary phase: Analyte-retention stability and application in the analysis of nonsteroidal anti-inflammatory drugs. J. Chromatogr. A. (2024) 1722: Article 464871.
- DeLano M, Walter TH, Lauber MA, Gilar M, Jung MC, Nguyen JM, Boissel C, Patel AV, Bates-Harrison A, Wyndham KD. Using Hybrid Organic-Inorganic Surface Technology to Mitigate Analyte Interactions with Metal Surfaces in UHPLC. Anal. Chem. (2021), 93: 5773–5781.
- Berthelette KD, DeLoffi M, Collins C, Kalwood J, Walter TH. Correlation Between the Adsorption of Acidic Analytes on Stainless Steel Columns and Their Ionic Charge. Waters Application Note. 720008792. April 2025.
- Smith K, Wilson I, Rainville P. Sensitive and Reproducible MassSpectrometry-Compatible RP-UHPLC Analysis of Tricarboxylic Acid Cycle and Related Metabolites in Biological Fluids: Application to Human Urine. Anal. Chem. (2021) 93, 1009–1015.
720009039, September 2025