
This is an Application Brief and does not contain a detailed Experimental section.
This application brief demonstrates to quickly assess the quality of vanilla beans by developing a process that includes extraction of vanilla beans with Waters MV-10 ASFE System, followed by quantitative analysis of the resulting extracts by SFC.
The MV-10 ASFE System increases productivity by reducing the process time necessary for routine quality control applications.
Vanilla, the world’s most popular flavor, is widely used in food and cosmetic industries, and ranks as the second most expensive spice. Commercial vanilla extract manufacturing employs percolation or maceration of vanilla beans in a water/ethanol mixture for 48 hours. Conceivably, the quality of vanilla beans has a direct impact on the flavor and content of the resulting vanilla extract. However, there is no regulation on the quality of vanilla beans. It is, therefore, important for manufacturers to have a fast and reliable method to assess the quality of vanilla beans prior to manufacturing.
Supercritical fluid extraction (SFE) has been adopted for commercial caffeine production from coffee beans. Using liquid CO2 as the primary extraction solvent, the gas-like high diffusivity and liquid-like solvation strength allow liquid CO2 to penetrate deep into the pores of the beans, dissolving the target compounds, and transport the dissolved compounds out of the pores. Here, we propose a supercritical CO2 based process, SFE, to quickly assess the quality of vanilla beans.
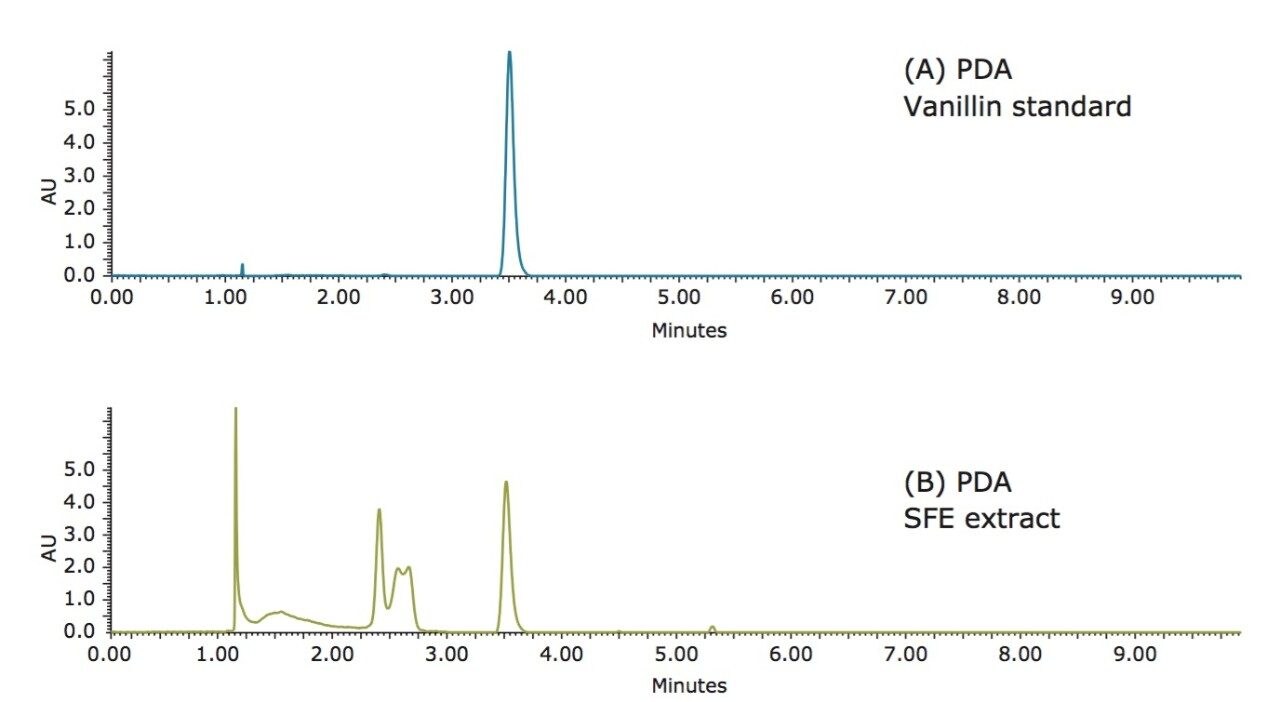
There are many compounds present in vanilla extracts, as shown in Figure 1. Among them, vanillin is the primary component responsible for the characteristic flavor and smell of vanilla. Vanillin was, therefore, chosen as the marker to assess the quality of vanilla beans. The chromatography was optimized to ensure that the vanillin peak was free from any interfering peaks for accurate quantitative analyses.
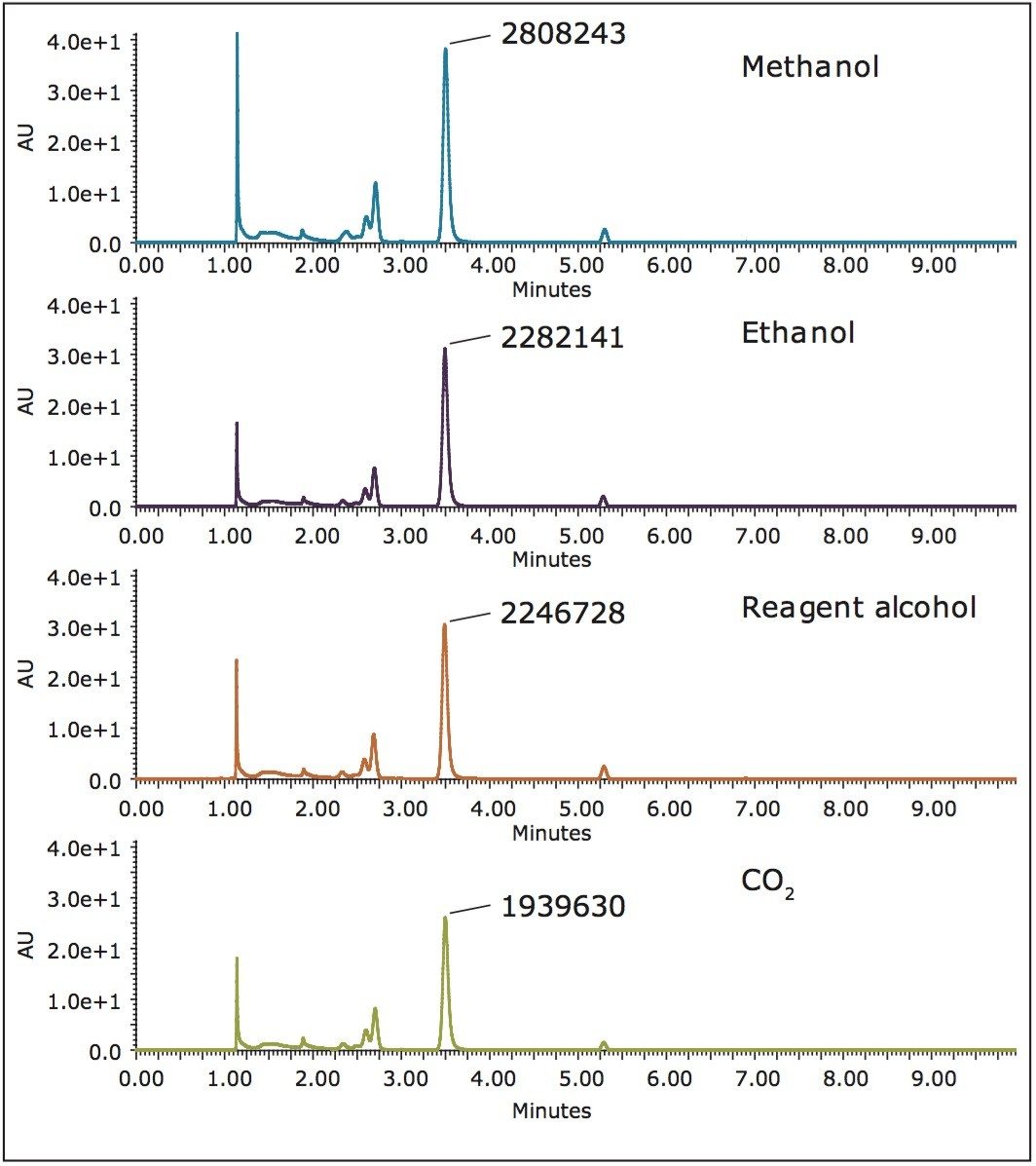
All extraction experiments were performed on a Waters MV-10 ASFE System. Multiple extraction vessels were loaded with sample, and placed in the MV-10 ASFE multi-vessel column oven. The sequence was then run in an automated fashion using ChromScope Software. Figure 2 shows the SFC/UV chromatograms of vanilla extracts using different co-solvents. The vanillin yield decreased as the polarity of the co-solvent decreased. This can be ascribed to the polarity of vanillin. Since vanillin is a relatively polar compound, it dissolves more in polar solvents such as methanol. Methanol was, therefore, chosen as the co-solvent for ensuing extractions; although, ethanol is the preferred solvent for commercial vanilla extract.
The optimized extractions included a 3-min dynamic extraction, a 10-min static soaking step, followed by a 30-min dynamic extraction at 300 bar and 40 °C, with 5% methanol as the co-solvent. The resulting extracts were then diluted by methanol and subject to supercritical fluid chromatography (SFC) analysis. Figure 3 shows the SFC/UV chromatograms of two types of vanilla beans from two different cultivars. Quantitative analyses revealed that there is a substantial difference in vanillin content between the two batches. Batch 1 contains 65% more vanillin than Batch 2. The total process time, including extraction by SFE and chromatographic analysis by SFC, was less than 1 h per sample.
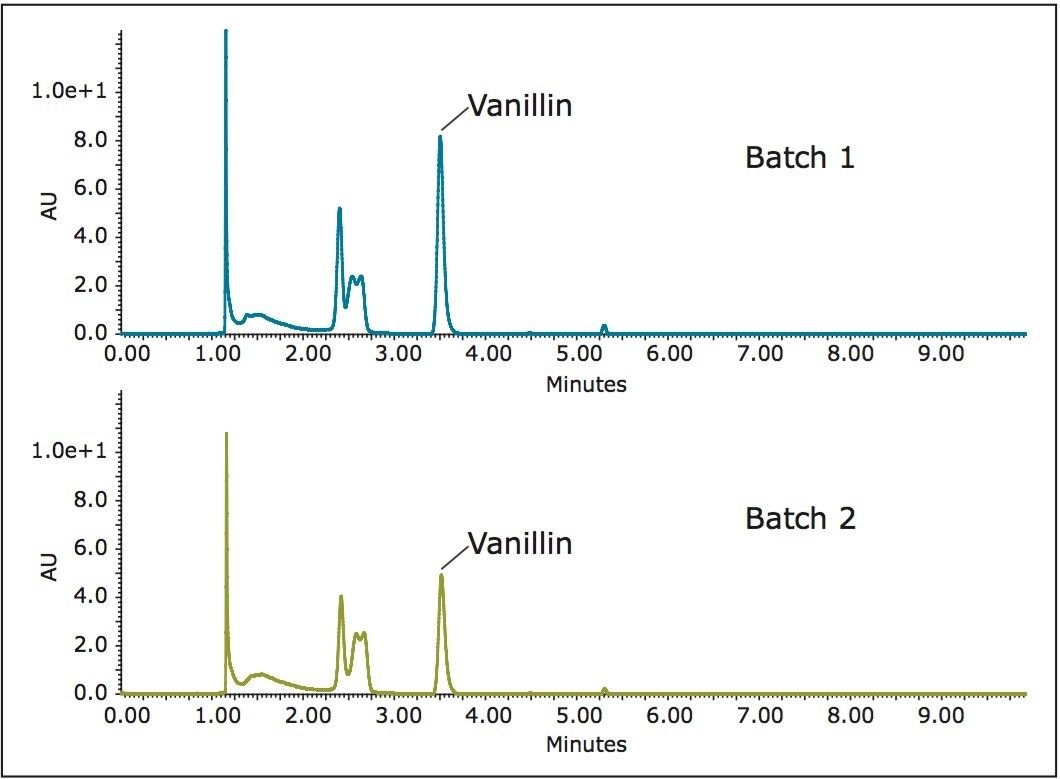
Two batches of vanilla beans from different cultivars were extracted using SFE. Compared to conventional extraction methods, SFE offers a much cleaner and efficient alternative. The resulting extracts were analyzed by SFC/UV. Using vanillin as a marker, quantitative analyses revealed that Batch 1 contained 65% more vanillin than Batch 2. The total process time, including extraction and chromatography, was less than 1 h. The MV-10 ASFE System minimizes raw material consumption and, as a result, solvent consumption. The multi-vessel extraction capability of the MV-10 ASFE System, along with ChromScope Software, increases lab productivity by enabling multiple sample batches to run in an automated fashion without user intervention.
720004276, May 2012