Simvastatin is a lipid-lowering agent that is derived synthetically from a fermentation product of Aspergillus terreus. The Uniformity of Content Assay for simvastatin is accomplished using high performance liquid chromatography (HPLC) with ultraviolet detection (UV). The faster that this assay can be performed, the faster the finished product can be released and revenue realized. In this application note, we show how the HPLC assay for simvastatin has been transferred to UPLC. The UPLC assay is compared to the USP assay criteria for performance and quality.
The final methodology reduced the analysis time from an analyte retention of 9.28 minutes with HPLC to an analyte retention of just 1.41 minutes with UPLC (a seven-fold increase in throughput).
The ACQUITY UPLC Console Calculator was used to easily guide the transfer methodology to ACQUITY UPLC using sub-2 µm particles. The calculator gave three separate options for UPLC methods: Equal Efficiency, Maximum Efficiency and Shortest Analysis Time conditions.
We demonstrate the ease of HPLC to UPLC method transferability, and the benefits that can be obtained in any time-, resource- and/or revenue-conscious laboratory environment where UPLC can significantly increase throughput with quality results. And with a variety of ACQUITY UPLC BEH Column dimensions, scientists have the flexibility to tailor their UPLC separations to the goals at hand.
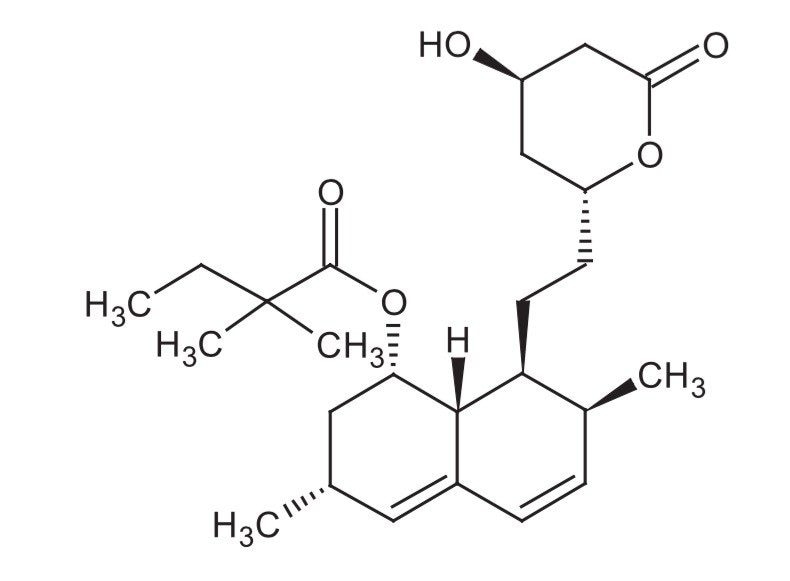
Simvastatin (Figure 1) is a lipid-lowering agent that is derived synthetically from a fermentation product of Aspergillus terreus. After oral ingestion, simvastatin (an inactive lactone) is hydrolyzed to the corresponding b-hydroxyacid form. This is an inhibitor of 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase. This enzyme catalyzes the conversion of HMG-CoA to mevalonate, which is an early and rate-limiting step in the biosynthesis of cholesterol.
Simvastatin is most commonly administered orally in a tablet containing either 5 mg, 10 mg, 20 mg, 40 mg or 80 mg of the active pharmaceutical ingredient (simvastatin) and the following inactive ingredients: cellulose, hydroxypropyl cellulose, hydroxypropyl methylcellulose, iron oxides, lactose, magnesium stearate, starch, talc, titanium dioxide and other ingredients. Butylated hydroxyanisole is added as a preservative.
The Uniformity of Content Assay for simvastatin is accomplished using high performance liquid chromatography (HPLC) with ultraviolet detection (UV). The faster that this assay can be performed, the faster the finished product can be released and revenue realized. The current USP 30–NF 25 monograph assay for simvastatin tablets calls for the use of a liquid chromatograph equipped with a 238 nm detector and a 4.6 mm x 25 cm column containing packing L1 maintained at a temperature of 45 °C, and a flow rate of about 1.5 mL per minute. The performance of the assay demands a capacity factor, k', of not less than 3.0. The column efficiency should not be less than 4,500 theoretical plates, with a tailing factor of not more than 2.0 and a relative standard deviation for replicate injections of not more than 2.0%. The current HPLC-based assay has an analysis time of 12 minutes, with a retention time of 9.28 minutes for the active ingredient simvastatin.
UltraPerformance LC is a new category of separation science which builds upon well-established principles of liquid chromatography, using sub-2 μm porous particles. These particles operate at elevated mobile phase linear velocities to produce rapid separations with increased sensitivity and increased resolution. Thus, UPLC technology allows analysis times to be dramatically reduced while still meeting assay acceptance criteria based on plate count, resolution and analyte retention. In this application note, we show how the HPLC assay for simvastatin has been transferred to UPLC. The UPLC assay is compared to the USP assay criteria for performance and quality.
A standard solution of simvastatin was prepared according to the USP methodology, and then was diluted to 100 μg/mL for chromatographic analysis.
|
LC System: |
Alliance HPLC System |
|
Column: |
XBridge C18, 5 μm, 4.6 x 250 mm |
|
Mobile Phase: |
65% acetonitrile/35% phosphate buffer, pH 4.5 |
|
Flow Rate: |
1.5 mL/min |
|
Injection Volume: |
10 μL |
|
Temperature: |
45 °C |
|
Detection: |
PDA @ 238 nm |
|
LC System: |
ACQUITY UPLC System |
|
Column: |
ACQUITY UPLC BEH C18, 1.7 μm, 2.1 x 100 mm, and 2.1 x 30 mm |
|
Mobile Phase: |
65% acetonitrile/35% aqueous |
|
Flow Rate: |
0.56 mL/min |
|
Injection Volume: |
0.8 μL |
|
Temperature: |
45 °C |
|
Detection: |
ACQUITY UPLC PDA @ 238 nm |
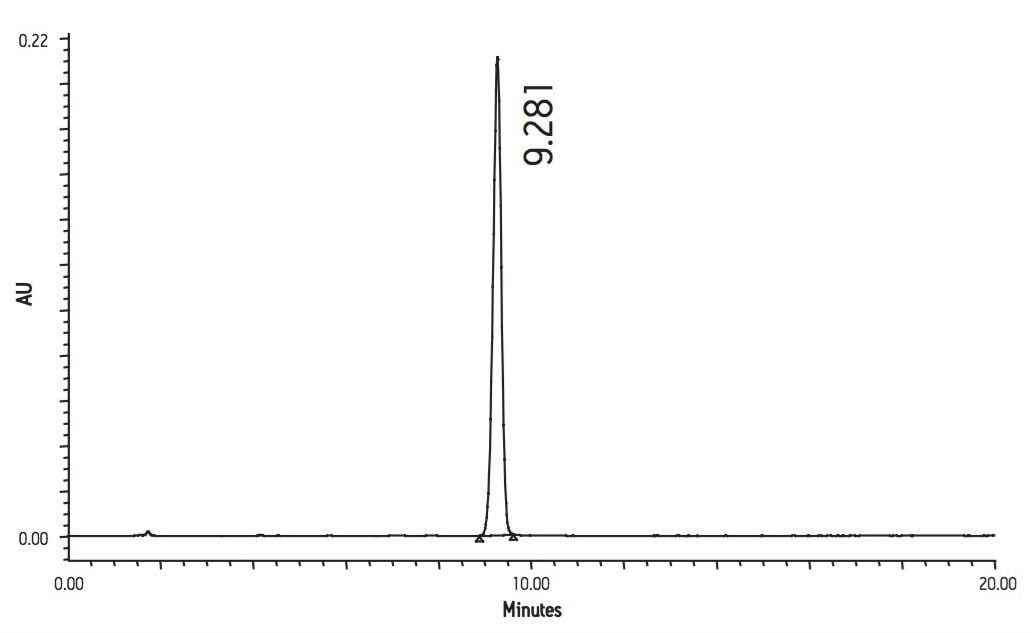
The HPLC separation of simvastatin is shown in Figure 2. Here we can see that the analyte was eluted with a retention time of 9.28 minutes. The HPLC analysis generated a k' value of 5.5, a 4σ plate count of 12,112 and a tailing factor of 0.94.

The HPLC method was scaled to UPLC using the ACQUITY UPLC Console Calculator. The calculator allows the user to input the current HPLC conditions, including column length, mobile phase composition and flow rate. The calculator then generates three results: 1.) the conditions of “Equal Efficiency,” 2.) the conditions of “Maximum Efficiency,” and 3.) the conditions of “Shortest Analysis Time.” The HPLC conditions for simvastatin were input and the results obtained are shown in Figure 3.
The data generated shows three results relating to three potential UPLC conditions. The calculator transfers the HPLC condition to UPLC, taking into account changes in column diameter and particle size when calculating the flow rate. The equivalent conditions are calculated by adjusting the flow rate by the ratio of 2.12/4.62 to account for column geometry, and then multiplied by the ratio of 5/1.7 to account for the reduction in particle size.
The injection volume was scaled to account for the change in overall column volume.
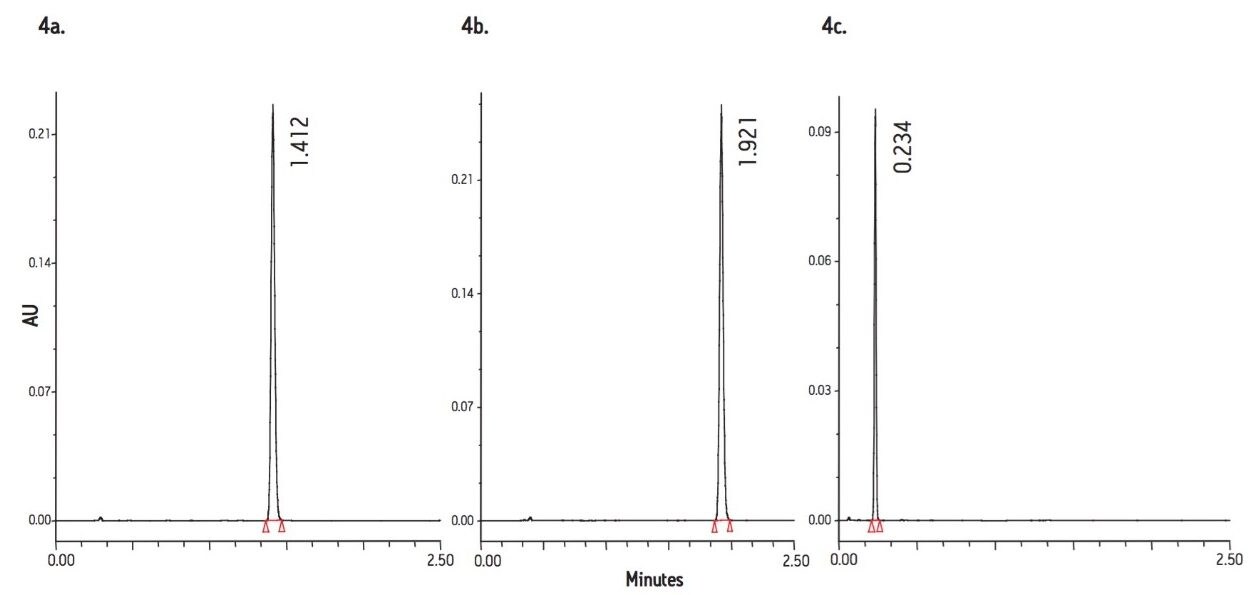
The transferred conditions of Equal Efficiency suggest the use of a 2.1 x 100 mm, 1.7 μm C18 column operating at a flow rate of 766 μL/min and an injection volume of 0.8 μL. As the injection volume suggested was very low, a 2 μL injection loop was installed in the autosampler. The data obtained from the use of these conditions is displayed in Figure 4a.
Results indicate an analysis time of 1.41 minutes. The ACQUITY UPLC System produced an efficiency of 12,874, a k' value of 4.88, and a USP tailing factor of 1.10. These values compare favorably with the original HPLC values of 12,112 for efficiency, 5.5 for k' and a tailing factor of 0.94.
The conditions of Maximum Efficiency required the use of a flow rate of 560 μL/min and an analysis time of 1.92 minutes. The data obtained using these conditions is displayed in Figure 4b. Here we can see that the peak retention was slightly greater than with the Equal Efficiency conditions. The chromatographic performance calculations revealed that the chromatographic efficiency increased to a value of 17,685, a k' factor of 5, and a tailing factor of 1.10.
The conditions of Shortest Analysis Time were performed on a 2.1 x 30 mm, 1.7 μm C18 column, at a flow rate of 1.5 mL/min. The data generated is displayed in Figure 4c. These conditions resulted in an analysis time of just 0.23 minutes; however, the efficiency count of this separation was below the USP acceptance criteria for this assay.
The method transfer was achieved by keeping L/dp constant; the initial conditions used a 250 mm column and a 5 μm particle, giving an L/dp value of 50 (250/5). In moving to a 2.1 x 100 mm, 1.7 μm C18 column, the L/dp value increased to 59, which allowed for a more efficient chromatography system. However, when the 2.1 x 30 mm, 1.7 μm C18 column was employed, the L/dp value was reduced to a value of 29; this is why the chromatographic efficiency did not meet the USP acceptance criteria.
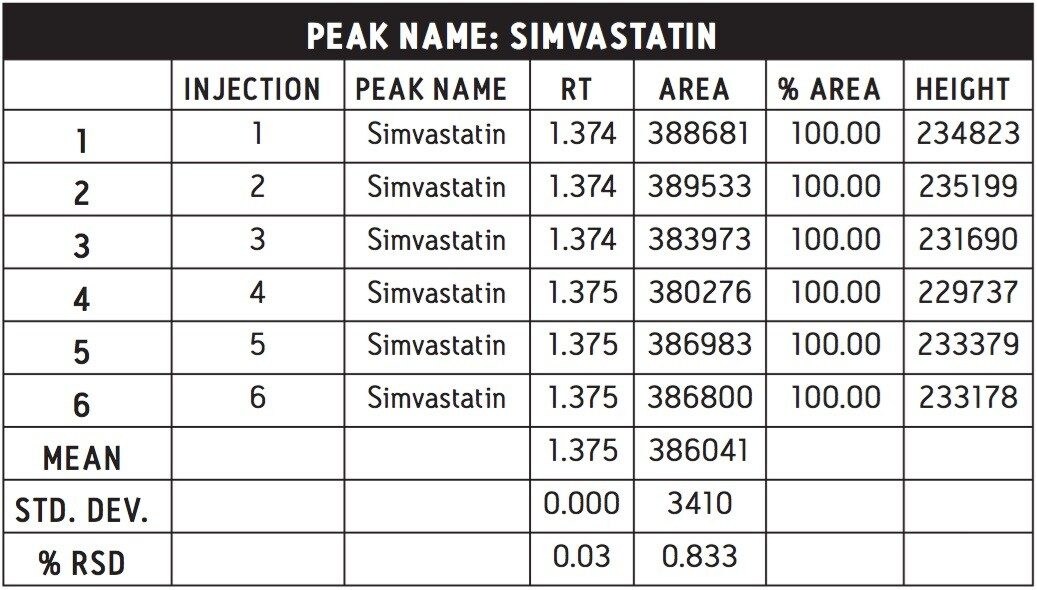
The UPLC assay selected for use and evaluation was the one employing Equal Efficiency conditions, as it yielded the highest throughput while meeting the assay acceptance criteria. The assay reproducibility performance was evaluated using the conditions developed by the console calculator. The results generated are shown in Table 1, where we can see that the percent RSD for this methodology was determined to be 0.883 for six 0.8 μL replicate injections of the simvastatin standard. These results were well within the USP acceptance criteria for the methodology.
The USP HPLC-UV methodology for the assay of the cholesterollowering drug simvastatin has been successfully transferred to an ACQUITY UPLC method. The final methodology reduced the analysis time from an analyte retention of 9.28 minutes with HPLC to an analyte retention of just 1.41 minutes with UPLC (a seven-fold increase in throughput). The ACQUITY UPLC Console Calculator was used to easily guide the transfer methodology to ACQUITY UPLC using sub- 2 μm particles. The calculator gave three separate options for UPLC methods: Equal Efficiency, Maximum Efficiency and Shortest Analysis Time conditions. The Equal Efficiency calculations gave an almost exact transfer of chromatographic performance when compared to the original HPLC conditions. With both the equal and maximum UPLC conditions, the USP assay criteria of efficiency, k' and tailing, were met or exceeded. The assay reproducibility performance was determined for the Equal Efficiency method and was found to be less than 1% RSD for six replicate injections. Finally, the assay was transferred to a Shortest Analysis Time method on the ACQUITY UPLC System which gave an analysis time of 0.3 minutes with an analyte retention of 0.23 minutes, resulting in an increase in throughput of 40-fold.
We have demonstrated the ease of HPLC to UPLC method transferability, and the benefits that can be obtained in any time-, resource- and/or revenue-conscious laboratory environment where UPLC can significantly increase throughput with quality results. And with a variety of ACQUITY UPLC BEH Column dimensions, scientists have the flexibility to tailor their UPLC separations to the goals at hand.
720001901, May 2007