This is an Application Brief and does not contain a detailed Experimental section.
This application brief demonstrates the benefits of UPLC compared to traditional HPLC for the analysis of risperidone, 9-hydroxyrisperidone, and the internal standard, clozapine.
HPLC-MS/MS is a widely utilized technique for quantitative bioanalysis due to its sensitivity and selectivity. There are three main challenges that face LC-MS/MS when developing new methods these are:
Waters solution to the above challenges is the ACQUITY UltraPerformance LC System.
|
HPLC system: |
Waters Alliance HT System |
|
Column: |
Xterra MS C18, 2.1 x 50 mm, 3.5 μm |
|
Mobile phase A: |
2 mM CH3COO–NH4+in H2O, pH 9.0 |
|
Mobile phase B: |
Methanol |
|
Flow rate: |
0.3 mL/min |
|
Injection volume: |
5 μL |
|
Sample diluent: |
0:50 v/v Methanol:Water |
|
Column temp.: |
50 °C |
|
Total run time: |
5.5 min. |
|
Time (min) |
A% |
B% |
Curve |
|---|---|---|---|
|
0.0 |
50 |
50 |
- |
|
0.5 |
50 |
50 |
6 |
|
2.0 |
0 |
100 |
6 |
|
2.5 |
50 |
50 |
11 |
|
UPLC system: |
Waters ACQUITY UPLC System |
|
Column: |
ACQUITY UPLC BEH C18, 2.1 x 50 mm, 1.7 μm |
|
Mobile phase A: |
2 mM CH3COO–NH4+in H2O, pH 9.0 |
|
Mobile phase B: |
Methanol |
|
Flow rate: |
0.6 mL/min |
|
Injection volume: |
5 μL |
|
Sample diluent: |
50:50 v/vMethanol:Water |
|
Column temp.: |
50 °C |
|
Total run time: |
1.5 min |
|
Time (min) |
A% |
B% |
Curve |
|---|---|---|---|
|
0.00 |
50 |
50 |
- |
|
0.25 |
50 |
50 |
6 |
|
0.75 |
0 |
100 |
6 |
|
1.25 |
50 |
50 |
11 |
|
MS system: |
Waters Micromass Quattro Premier XE |
|
Ionization mode: |
Positive Ion Electrospray (ESI+) |
|
Capillary voltage: |
3.00 V |
|
Desolvation temp.: |
380 °C |
|
Desolvation gas flow: |
800 L/hr |
|
Cone gas flow: |
50 L/hr |
|
Collision cell pressure: |
3.50e–3 |
|
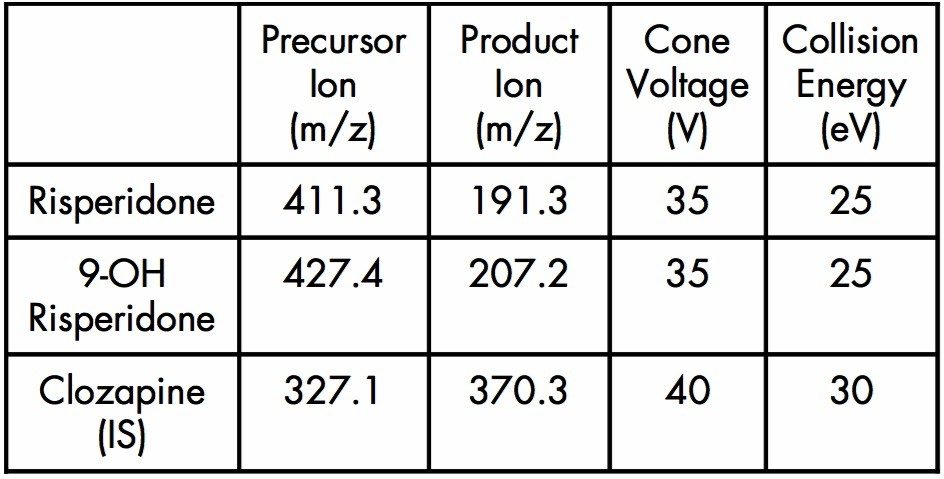
MRM transitions: |
Dwell Time: 30 ms for all transitions Inter-Scan Delay: 10 ms for all transitions |
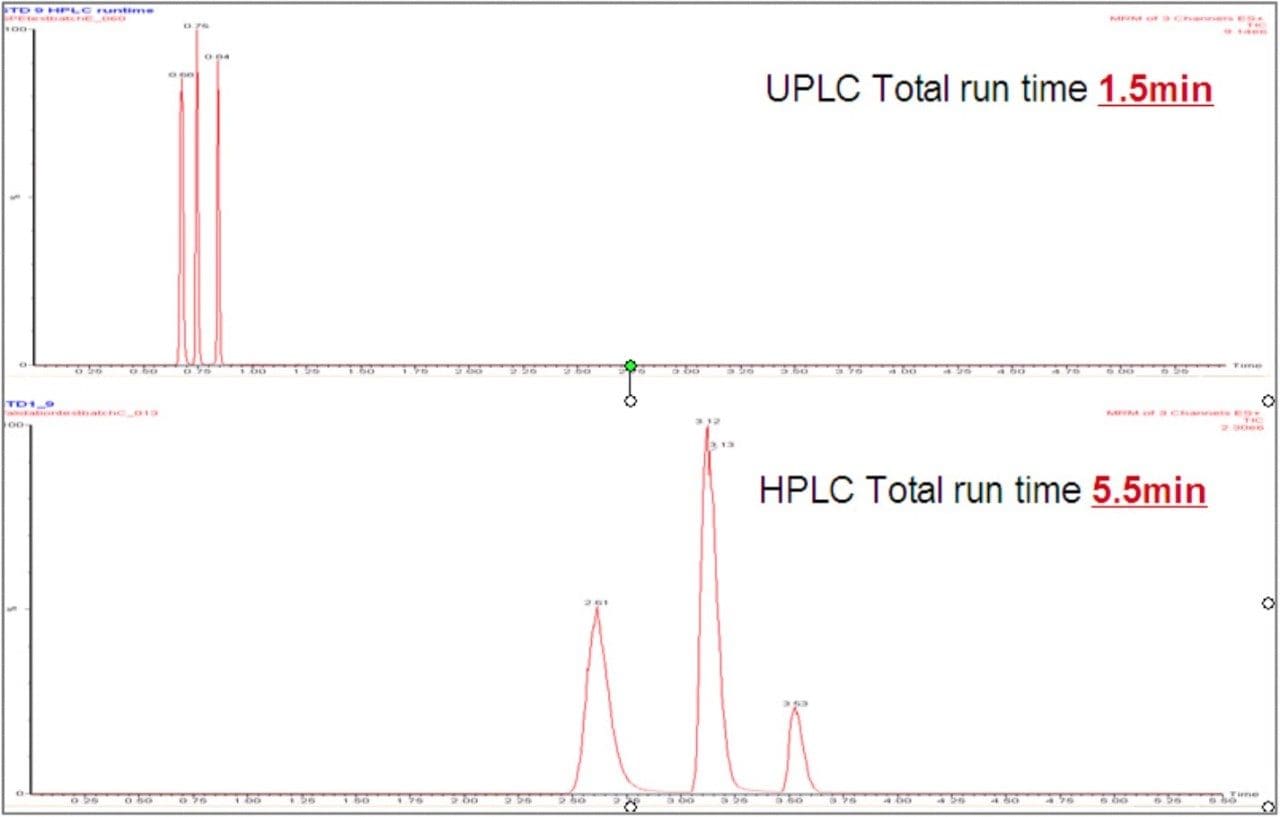
The UPLC method that was developed resulted in a 70% decrease inanalysis time compared to HPLC (Figure 1) with no significant change in chromatographic resolution (Figure 2), allowing a three-fold increase in sample throughput. The gradient and flow rates were optimized for both HPLC and UPLC and the reduction in analysis time results partly from the use of these conditions with the UPLC column, and partly to the very low system volume in the UPLC hardware. This low system volume also has a benefit in reducing the time required for equilibration when gradient elution is used, therefore further increasing sample throughput and allowing the efficient use of MS/MS.
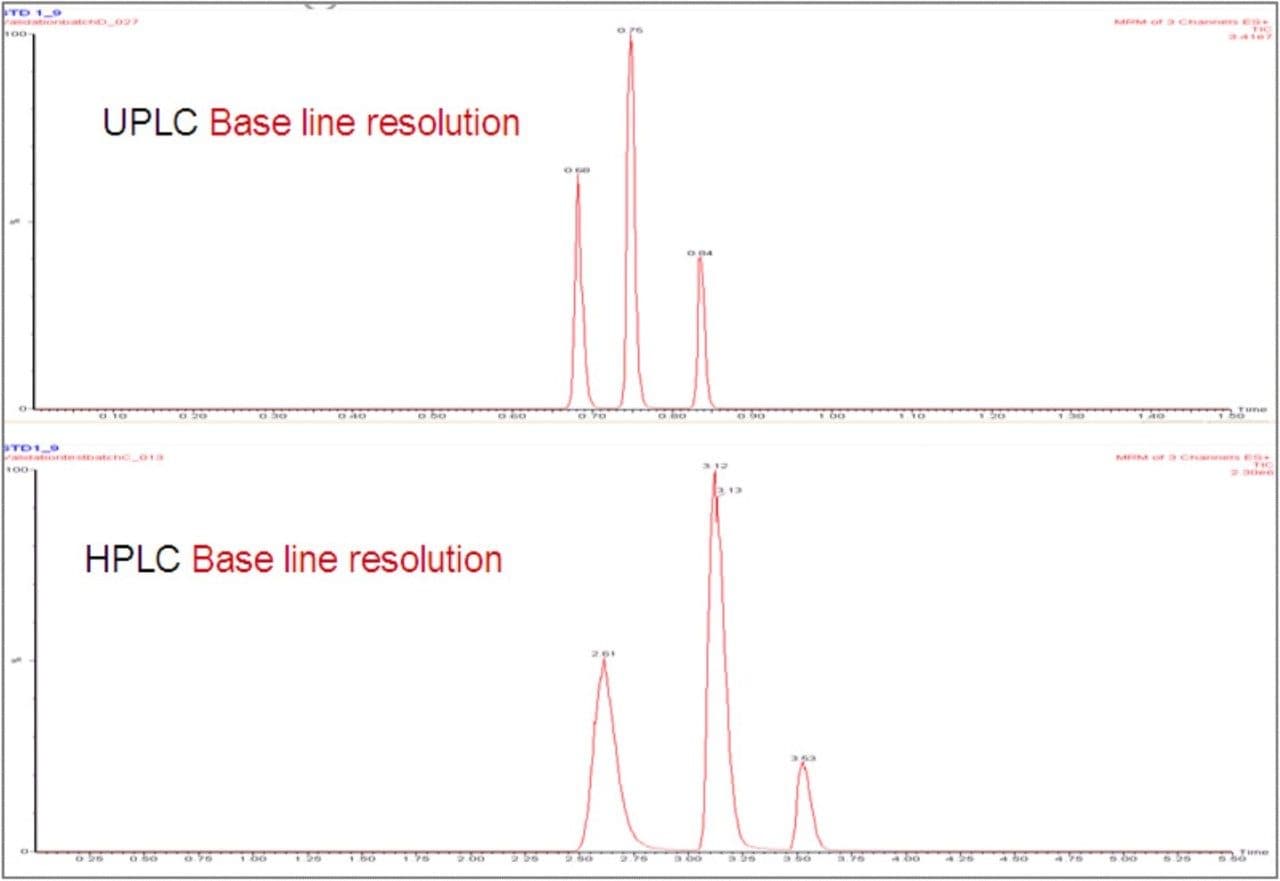
The narrow peak widths produced by the UPLC, typically 2–3 seconds wide at base, result in increased peak intensity and improved signal-to-noise ratios. This allows lower limits of quantification (LLOQ) to be reached compared to HPLC. In this example (Figures 3 and 4), a 3-fold increase in the signal-to-noise was achieved for a 0.1 ng/mL LLOQ.
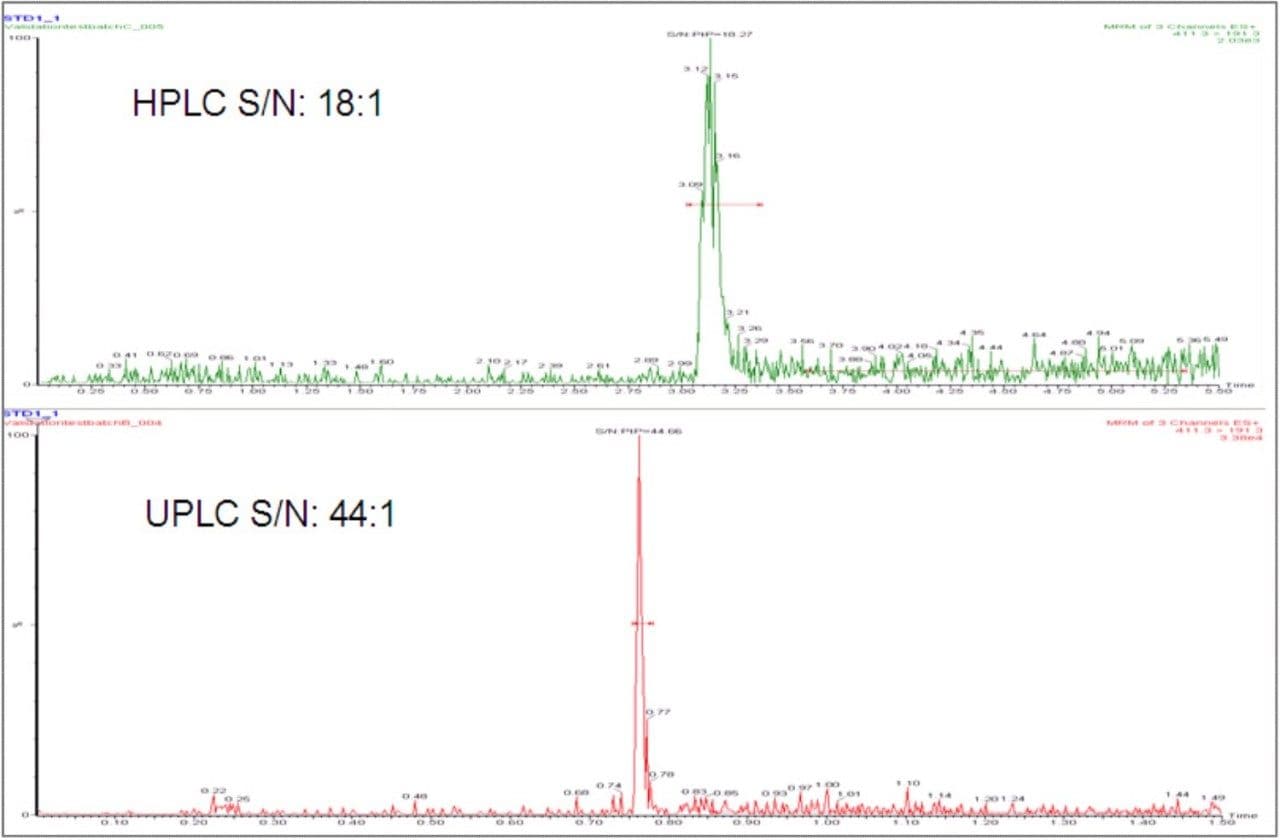
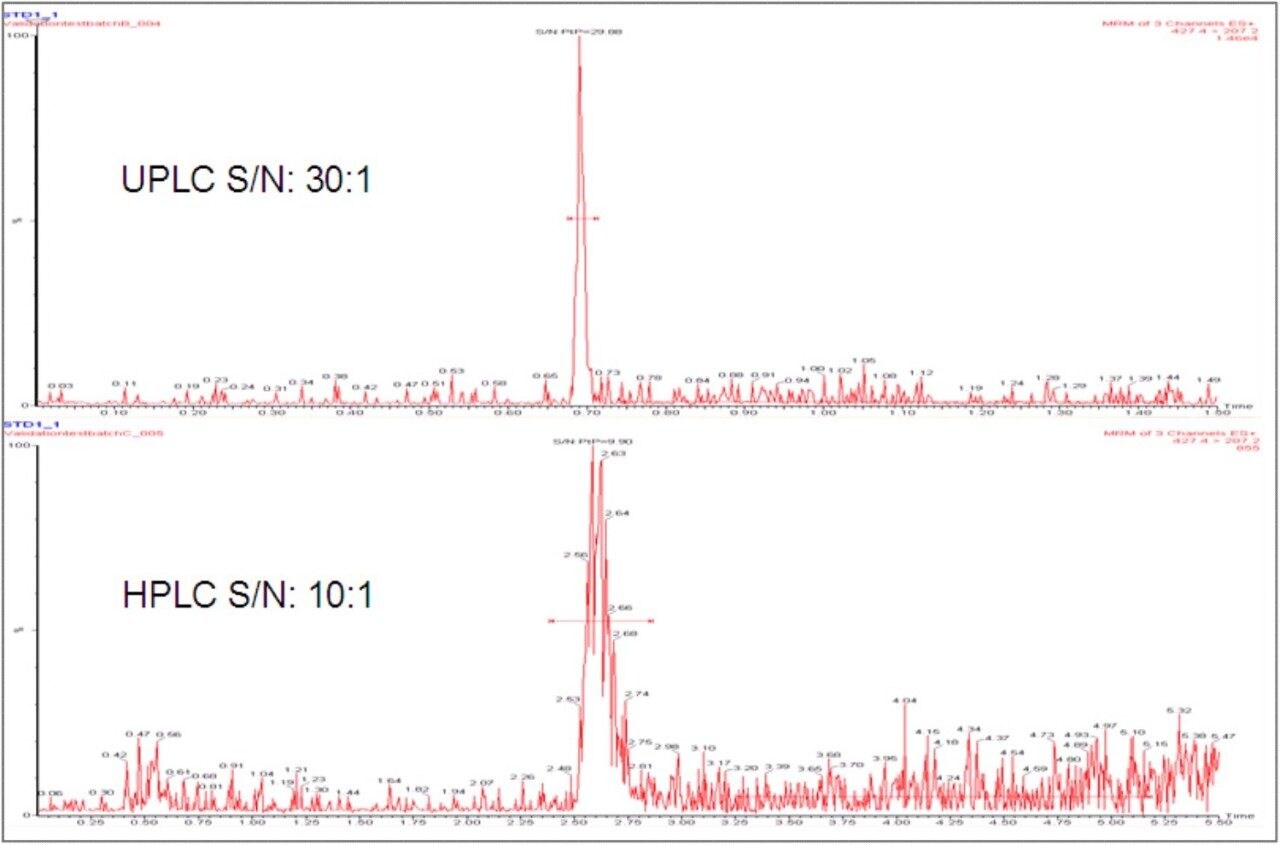
UPLC allows the development of fast and sensitive LC-MS/MS methods. When compared to conventional HPLC, significant reductions in analysis time and lower limits of quantification can be achieved, without the need to change the sample preparation method or the MS/MS system being used. In this example, a three-fold increase in both throughput and sensitivity was gained using the ACQUITY UPLC System.
720001443, December 2005