Chromatography Column Frits with MaxPeak High Performance Surfaces Prevent Nonspecific Binding of Nucleic Acids
Siqi Ji, Abraham S. Finny, Balasubrahmanyam Addepalli, Szabolcs Fekete, Jamuna Vaishnav, Kennedy Sawyer, Lavelay Kizekai, Mathew DeLano, Mingcheng Xu, Matthew Lauber
Waters Corporation, United States
Published on September 12, 2025
Abstract
The undesired adsorption of biomolecules to the porous frits that hold a chromatographic stationary phase at the inlet and outlet of a column has long been a problem in the field of chromatography. Such nonspecific adsorption is especially problematic for charged biomolecules and biotherapeutics including nucleic acids, proteins, viral vectors, etc. This phenomenon negatively impacts chromatographic performance by reducing recovery, altering peak shapes, and being an overall hindrance to achieving sensitive detection and accurate quantification.
In this application note, frits comprised of different materials were examined. Flow injection testing was performed using a 23 kilobase pair (kbp) dsDNA ladder. Degraded performance was noticed on traditional stainless steel (SS) frits with a recovery of 69% from the initial injection, which demonstrated the nonspecific adsorption of 23K dsDNA ladder to SS frits. This performance deteriorated further to 20% when testing was performed at higher back pressure (~9,000 psi). On the other hand, MaxPeak™ High Performance Surfaces (HPS) frits comprised of hybrid organic/inorganic surface on a corrosion resistant metal exhibited 100% recovery of the 23K dsDNA ladder indicating complete elimination of nonspecific adsorption under the given test conditions. Interestingly, both 0.2 µm and 0.5 µm porosity grade MaxPeak HPS frits produced equally high recoveries of the DNA molecules, suggesting that both grades exhibit permeability suitable for large nucleic acid separations.
Benefits
- Complete recovery of a 23K dsDNA ladder from MaxPeak HPS frits
- MaxPeak HPS frits are corrosion-resistant and modified with a hybrid organic/inorganic surface to effectively prevent nonspecific binding of biomolecules
- MaxPeak HPS frits of 0.2 µm and 0.5 µm porosity grades are both suitable for separations of large nucleic acids
Introduction
Chromatographic analysis is one of the most commonly employed analytical methods for the characterization and quantitation of biotherapeutic drug products including nucleic acids, proteins, viral vectors, etc. As these biomolecules become increasingly complex and higher in molecular weight, it becomes increasingly important to preserve analyte integrity and maximize recovery during chromatographic studies.
A frequently encountered yet often overlooked source of analyte loss is nonspecific adsorption to column hardware surfaces. While stationary phase chemistry is typically optimized for its intended use, other metallic components—including injector, tubing, flow cells, and frits—are sometimes neglected as sources of performance issues. Column frits, in particular, are prone to nonspecific interactions due to their high surface area (from porosity) and placement at points of maximum flow velocity. These characteristics make them hotspots for undesired interactions, particularly with large, ionic, and surface-active macromolecules.1
This issue is particularly severe when using SS frits, which can bind DNA and other charged biomolecules, resulting in distorted peak shapes, reduced recovery, and elevated back pressures. Traditional approaches to reduce nonspecific binding, such as introducing sacrificial analytes to saturate adsorption sites or adding mobile phase additives, are often time-consuming, wasteful, and can interfere with analyte detection. A more effective strategy has since emerged: carefully selecting frit materials and surface chemistries to minimize analyte-hardware interactions without compromising structural integrity or chromatography performance.2
To further explore this, the impact of frit composition and surface chemistries have been examined on the recovery of components in a 23K dsDNA ladder. This study compares SS frits with frits comprised of Waters™ MaxPeak HPS Technology. Additionally, different frit porosities and back pressures were assessed. This work highlights the importance of frit design in bioseparations and demonstrates the effectiveness of MaxPeak HPS frits.
Experimental
Lyophilized 23K dsDNA Ladder was obtained from Waters (p/n: 186011286) and resuspended in 100 µL of TE buffer (10 mM Tris-HCl, 1 mM EDTA; pH 8.0) to obtain a concentration of 500 µg/mL. The dsDNA species in this sample range from 125 bp to 23,130 bp in length. The stock dsDNA solution was diluted 100x (to obtain 5 µg/mL) to investigate the sensitivity of detection. Milli-Q® water was chosen as the mobile phase and dilution solvent to exacerbate the nonspecific adsorption of dsDNA.
Two frits made of the same materials and surface chemistries were first inserted into two 4.6 mm end nuts and then screwed tightly with specially designed frit testers. Two frits were connected in series with various tubing options to investigate the nonspecific binding phenomenon and will be referred to as a two-frit assembly (see Figure 1). Stainless steel (SS) 0.2 µm grade frits were tested alongside 0.2 µm and 0.5 µm grade hydrophilically modified MaxPeak HPS frits.
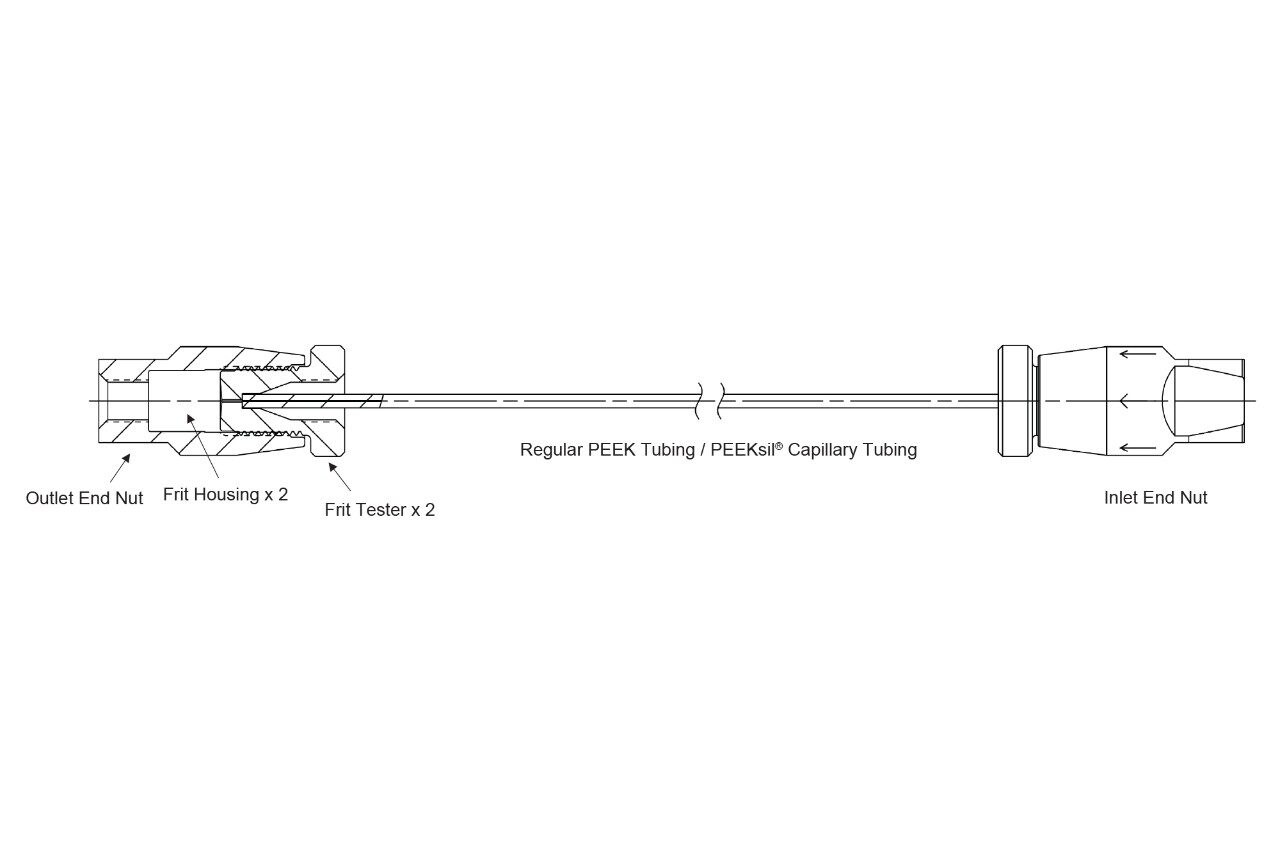
To test high back pressure effect, capillary tube was purchased from Trajan Scientific and Medical (PEEKsil™ tubing 1/16″ OD x 0.025 mm ID x 200 mm length, Catalog #: 14010102000102), assembled with a nut (IDEX, Catalog #: U-410X) and a ferrule (IDEX, Catalog #: U-401X) at both ends, and then connected in between two frits. PEEK tubing assembly with 0.025" ID and 8.5" length (Waters, p/n: 700009971) was used for connection to test under normal pressure conditions. No column or stationary phase was used in this study to mainly focus on the potential adsorption on the frits. The specific LC conditions are shown below, with the flow path of this system being passivated through repeat sample injections prior to the beginning of test experiments.
LC Conditions
|
LC system: |
ACQUITY™ UPLC™ H-Class Bio System |
|
Detection: |
ACQUITY TUV Detector |
|
Wavelength: |
260 nm |
|
Vials: |
QuanRecovery™ with MaxPeak HPS 12 x 32 mm Screw Neck Vial 300 µL Volume (p/n: 186009186), with Black Cap and Preslit PTFE/Silicone Septum (p/n: 186005827) |
|
Two-frit assembly: |
Two sets of frits in end nuts and frit testers, connected in series with PEEK tubing or capillary PEEKsil tubing |
|
Column heater temperature: |
30 °C |
|
Sample manager temperature: |
6 °C |
|
Injection volume: |
0.5 µL (23K dsDNA ladder, 5 µg/ml ) |
|
Flow rate: |
0.2 mL/min |
|
Mobile phase A: |
18.2 MΩ·cm (Milli-Q water) |
|
Mobile phase B: |
Frit storage solution (10% acetonitrile in 18.2 MΩ·cm water) |
|
Mobile phase C: |
70% Isopropanol in 18.2 MΩ·cm water |
|
Mobile phase D: |
70% Isopropanol in 18.2 MΩ·cm water |
|
Gradient: |
Isocratic run with 100% Mobile Phase A |
Data Management
|
Chromotography software: |
Empower 3 |
Results and Discussion
Normal Pressure Injection
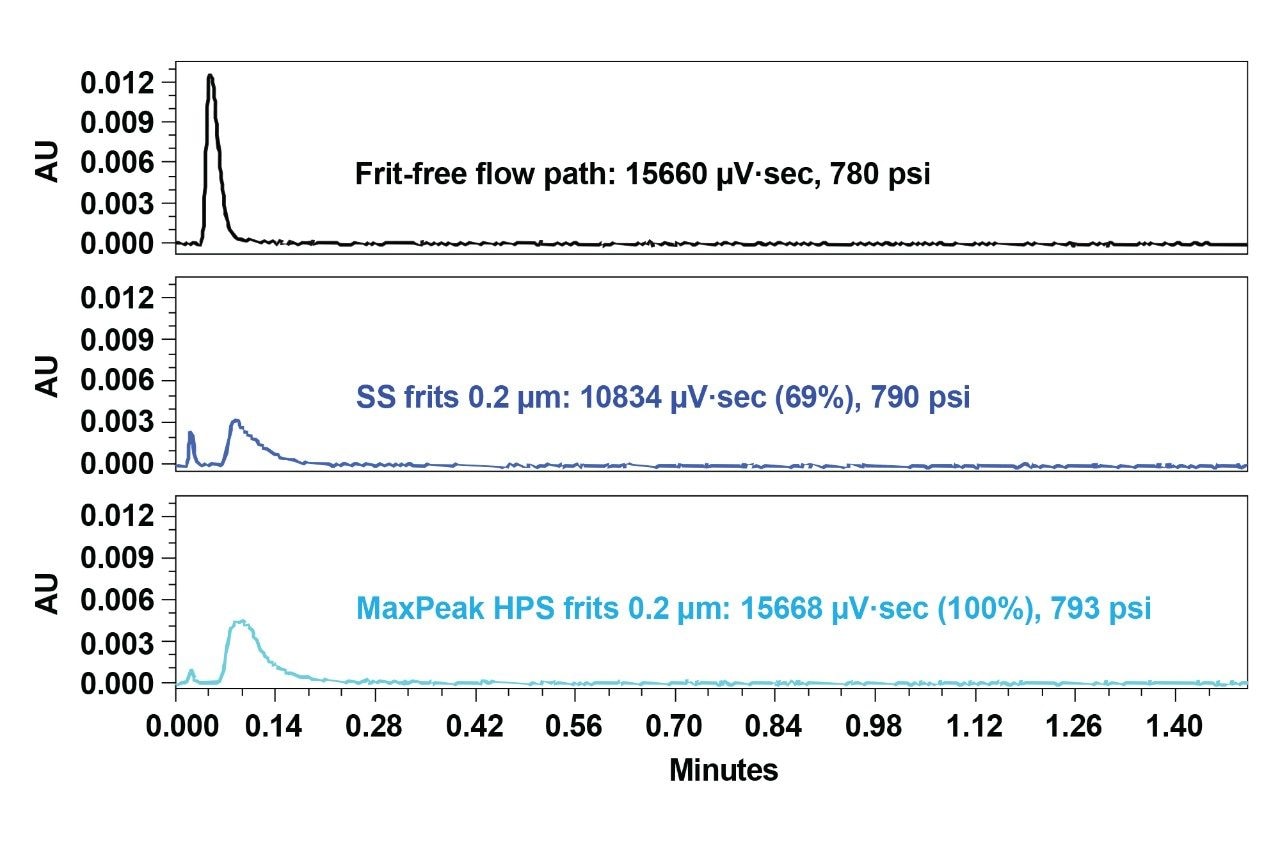
Initially, all of the chromatography column frits were tested at a relatively low pressure (~790 psi) and the observed optical signal at A260 nm was compared against an equivalent frit-free assembly to comparatively measure analyte recoveries. The porosity grade of frits in this first round of testing was 0.2 µm. Recovery was calculated according to the peak area ratio of the frits versus frit-free analysis. The signal values from the first injections were initially compared as shown in Figure 2. The SS frits that are traditionally used with chromatographic columns exhibited a recovery of 69%. Interestingly, 100% recovery was achieved with the MaxPeak HPS frits, indicating the positive impact of a more purposefully designed option.
High Pressure Injections
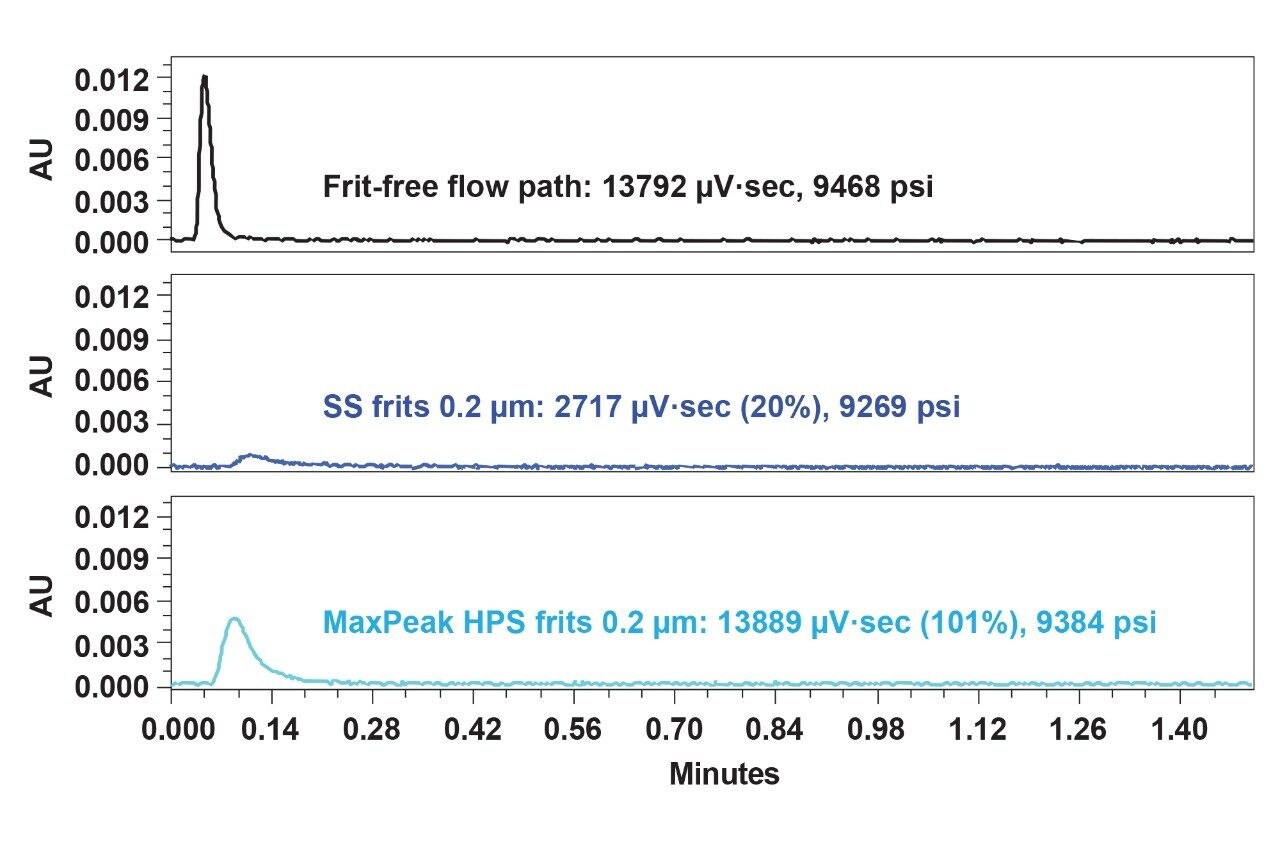
Shear force generated by high back pressure (~10,000 psi) is required for slalom chromatography-based separations. Here, the large dsDNA molecules undergo stretching by mobile phase induced shear stress leading to flow retardation. The time to relax depends on the size and shape of the nucleic acids.3 Because this chromatographic condition is so unique, it was chosen to be investigated in further detail. To simulate high pressure conditions, capillary PEEKsil tubing (0.025 mm ID) was introduced between the two frits to generate high pressure in the new assembly during 23K dsDNA injections. Figure 3 shows the chromatographic results for the first injection through the frit-free and the two-frit, high pressure assembly (which was devised to mimic a fully constructed column). The porosity of all frits was 0.2 µm to be consistent. Significantly, SS frits showed only 20% recovery when the back pressure was around 9269 psi, which is a 3.5X drop in recovery compared to low pressure test conditions. MaxPeak HPS frits were not influenced by the high-pressure effect, with a recovery of ~100%.
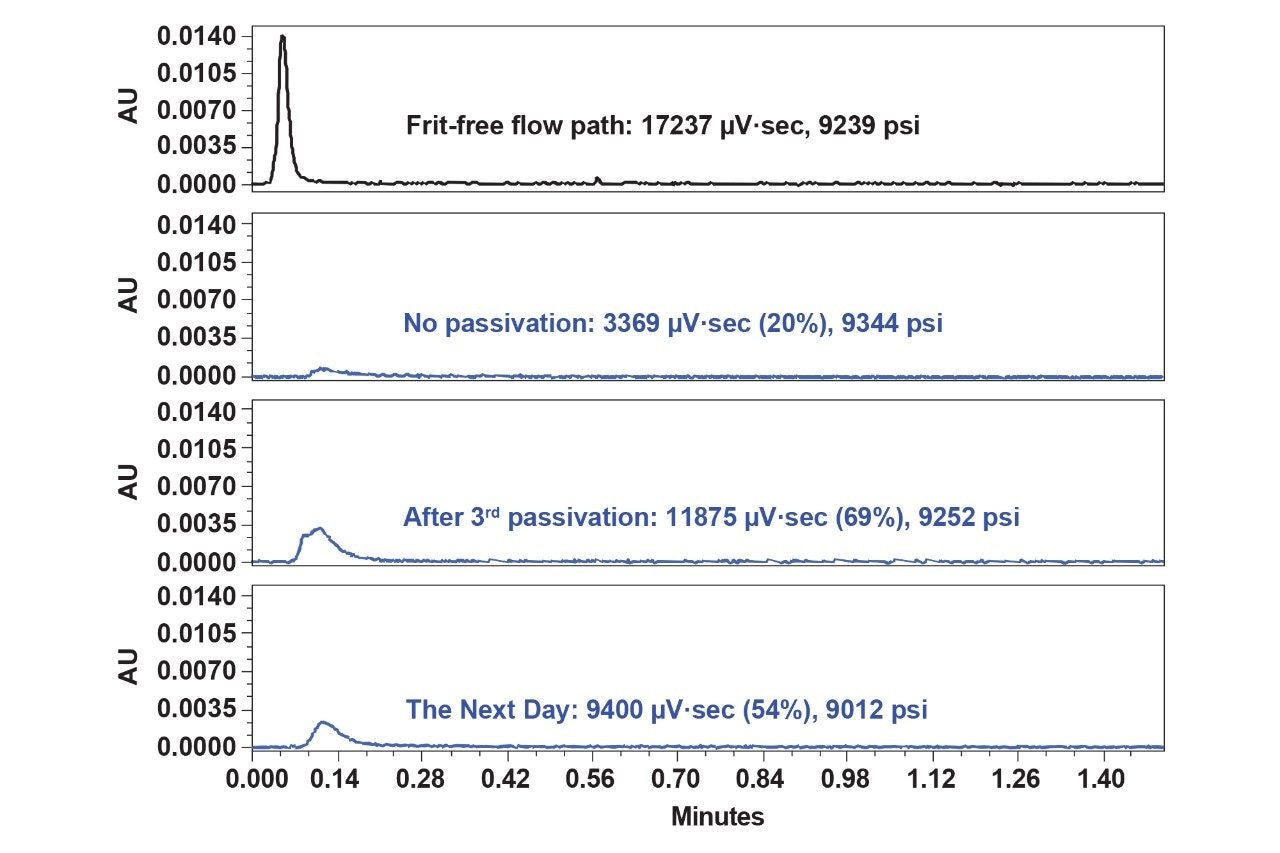
To further investigate the extremely low recovery of SS frits, thyroglobulin (10 mg/mL) was used as blocking or passivation agent. After three injections (1 µL ea.) of the passivation agent, the 23K dsDNA injection showed increased recovery (from 20% to 69%). This effect is shown in Figure 4. More than three passivation steps did not lead to any additional improvements in 23K dsDNA recovery. The recovery, however, dropped to 53% on the following day of testing. This data indicates low consistency and poor reliability of sample-based passivation techniques.
Porosity Effect
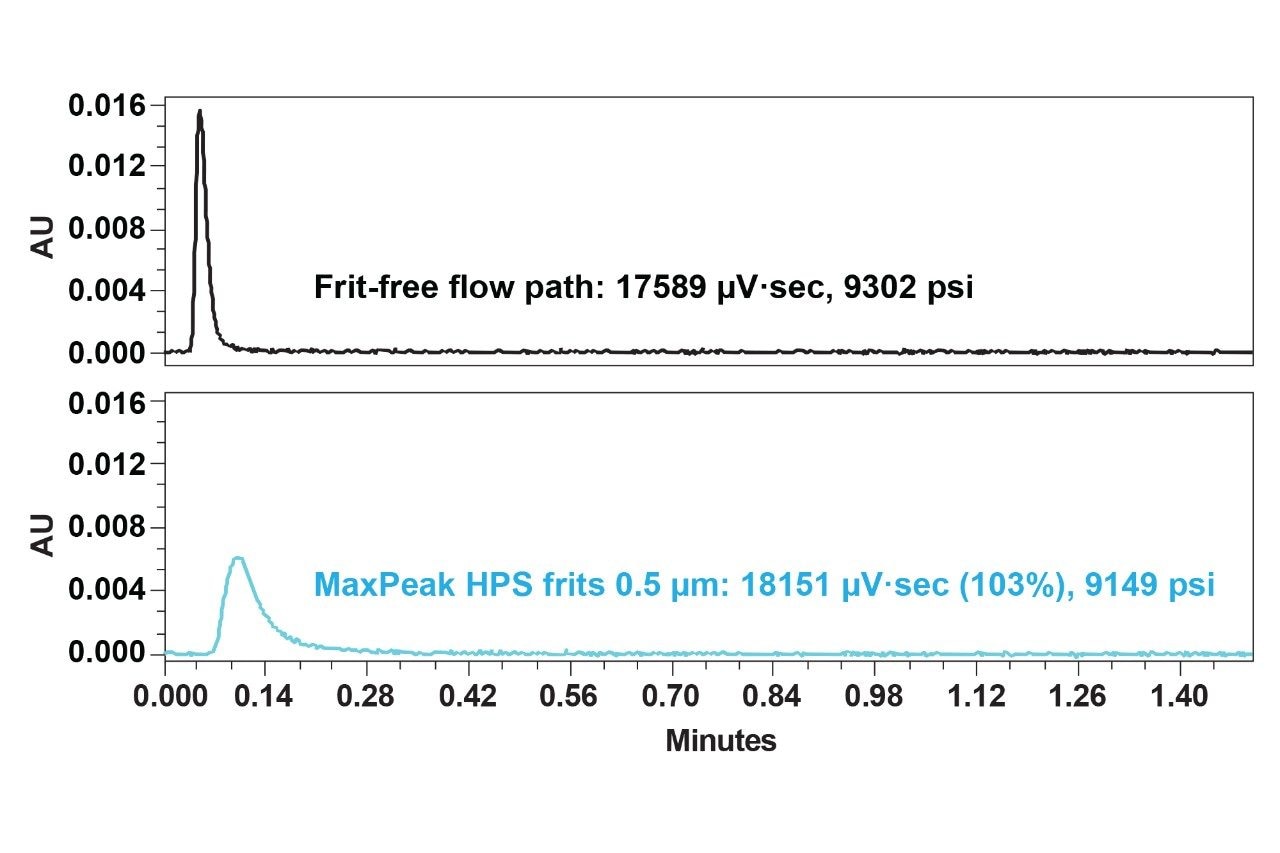
To compare porosity effects, MaxPeak HPS 0.5 µm grade frits with flow injections of the 23K dsDNA ladder was also tested under high pressure conditions. These frits are manufactured such that they have larger diameter channels versus the 0.2 µm grade frits. It can be concluded from Figure 5 that a 103% recovery was achieved by MaxPeak HPS 0.5 µm grade frits. The 103% recovery in chromatography could be within acceptable limits depending on the variations of tailing effects and integration parameters. As a result, it can be concluded that porosity does not influence the nonspecific binding of large nucleic acid species on MaxPeak HPS frits, at least when studying nucleic acid species limited to 23K in size.
Conclusion
In this application note, a comparison was made on the nonspecific binding of analytes on SS frits versus MaxPeak HPS frits through the implementation of flow injection testing using 23K dsDNA ladder. SS frits performed poorly under high pressure (~10,000 psi) conditions, showing recovery values as low as 20% for this large dsDNA sample mixture. Thyroglobulin passivation could improve nucleic acid recoveries to some extent (up to 69%) but the recoveries are inconsistent with poor reproducibility. Frits with 0.2 µm and 0.5 µm porosity grade specifications were compared as well, showing no obvious effects when manufactured as MaxPeak HPS variants. In conclusion, the hydrophilically-modified MaxPeak HPS frits showed excellent performance with 100% recoveries for large dsDNA species. This confirms their suitability for new large nucleic acid separations, such as Waters GTxResolve™ 2000 Å SEC and Slalom Chromatography Column.
Waters, MaxPeak, ACQUITY, UPLC, QuanRecovery and GTxResolve are trademarks of Waters Technologies Corporation. Milli-Q is a trademark of Merck KGaA. PEEKsil is a trademark of Trajan Scientific Australia Pty Ltd.
References
- J. Simeone et al. “Optimizing test approaches for the detection of exposed metal surfaces within a chromatographic flow path,” J Chromatogr A, vol. 1666, p. 462855, 2022, doi: https://doi.org/10.1016/j.chroma.2022.462855.
- G. J. Guimaraes and M. G. Bartlett. “Managing nonspecific adsorption to liquid chromatography hardware: A review,” Anal Chim Acta, vol. 1250, p. 340994, 2023, doi: https://doi.org/10.1016/j.aca.2023.340994.
- F. Gritti. “Ultra-high pressure slalom chromatography: Application to the characterization of large DNA and RNA samples relevant in cell and gene therapy,” J Chromatogr A, vol. 1738, p. 465487, 2024, doi: https://doi.org/10.1016/j.chroma.2024.465487.
Acknowledgement
We would like to thank Steven Byrd and Lili Macedo for bringing the H-class system back online, performing a 6-Month Cleaning and Diagnostic Service. We also acknowledge the guidance from Frank Marszalkowski and Micah Watt to acquire different hardware from the Engineering lab and the manufacturing team, as well as to submit special requests to the assembly team. Special thanks to Anna Boardman, Christian Reidy, Rebecca Barnes, and the Content Operations team for assistance with the final proofreading and insightful suggestions that enhanced the overall presentation of this work.
720009020, September 2025