This is an Application Brief and does not contain a detailed Experimental section.
For in vitro diagnostic use. Not available in all countries.
This application brief demonstrates the metrological traceability of the product calibrators and controls, and the measurement accuracy of the Waters MassTrak Vitamin D Kit.
Metrological traceability of the MassTrak Vitamin D Kit has been established, consistent with ISO 17511. This aids laboratories with their compliance to ISO 15189.
The MassTrak Vitamin D Kit is designed for the quantitative determination of serum or plasma 25-hydroxyvitamin D3 (25OHD3) and 25-hydroxyvitamin D2 (25OHD2), which in combination provide the total 25-hydroxyvitamin D concentration as an aid in the assessment of vitamin D sufficiency. Metrological traceability has been incorporated in to the design, development, and manufacturing of the MassTrak Vitamin D Kit to meet the requirements of ISO 17511:2003 In vitro diagnostic medical devices – Measurement of quantities in biological samples – Metrological traceability of values assigned to calibrators and control material, which aids laboratories with their compliance to ISO 15189:2012 Medical laboratories – Requirements for quality and competence.
The accuracy of the reagent kit calibrators is further assured through continuing participation in the Centers for Disease Control and Prevention (CDC) Vitamin D Standardization-Certification Program (VDSCP) for total 25OHD.
In this technology brief, the MassTrak Vitamin D Kit metrological traceability is explained, and accuracy of the kit demonstrated by the results of the VDSCP.
The MassTrak Vitamin D Kit calibrators and quality control sets are traceable to the National Institute of Standards and Technology (NIST) Standard Reference Material (SRM) 2972 through an unbroken chain of calibrations as shown in Figure 2.
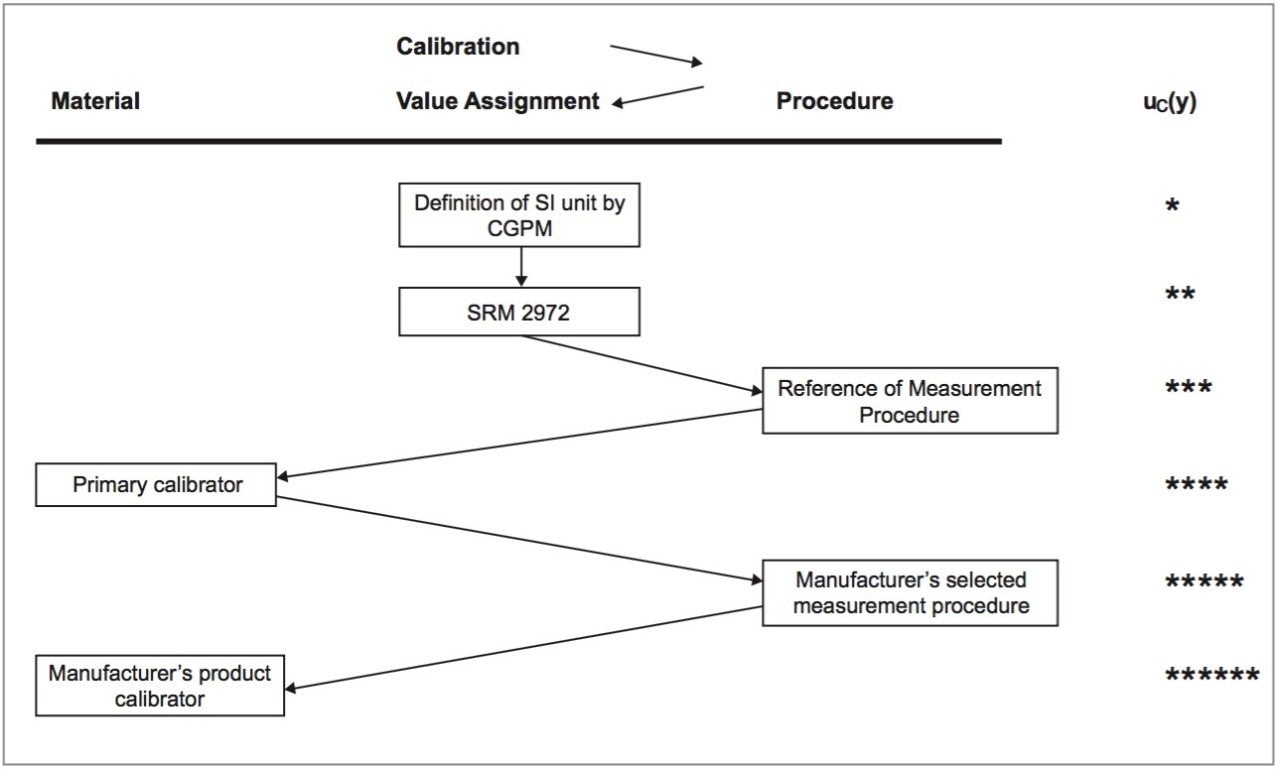
Traceability to NIST SRM 2972 is achieved through assignment of primary calibrators, using reference measurement procedures, which are used to value assign 25OHD2 and 25OHD3 concentrations to each lot of MassTrak Vitamin D Kit calibrators and quality control sets. The reference measurement procedures are listed in the Database of the Joint Committee for Traceability in Laboratory Medicine (JCTLM) at www.bipm.org (Reference Measurement Procedures; C8RMP3 and C8RMP4) and were performed at the Laboratory of Analytical Chemistry (University of Ghent), directed by Prof. Dr. Linda Thienpont.1,2
All MassTrak Vitamin D Kit calibrators and quality control sets are value assigned using these primary calibrators and the manufacturers’ routine measurement procedure (LC-MS/MS). The concentration values assigned to the MassTrak Vitamin D Kit calibrators and quality controls are verified in a value confirmation process. The value confirmation of the calibrators involves the measurement of independently assigned quality control material.
The uncertainty of the reference materials used and the uncertainty measurements for the MassTrak Vitamin D Kit calibrators are defined in the MassTrak Vitamin D Kit Directions For Use (715004830IVD).
To assess the accuracy and precision of the MassTrak Vitamin D Kit assay, Waters enrolls in the VDSCP for total serum 25OHD. The program assesses bias and precision of assays relative to reference measurement procedures.
The VDSCP Certification phase consists of ten blinded patient samples distributed quarterly. Each quarter the ten samples are analyzed on two days in duplicate for an annual total of 160 individual results. Measurement procedures meeting the VDSCP Certification Program’s acceptance criteria – overall mean bias (±5.0%) and precision (≤10.0%) for total Vitamin D, calculated following CLSI EP9-A2 – are considered certified by the program.
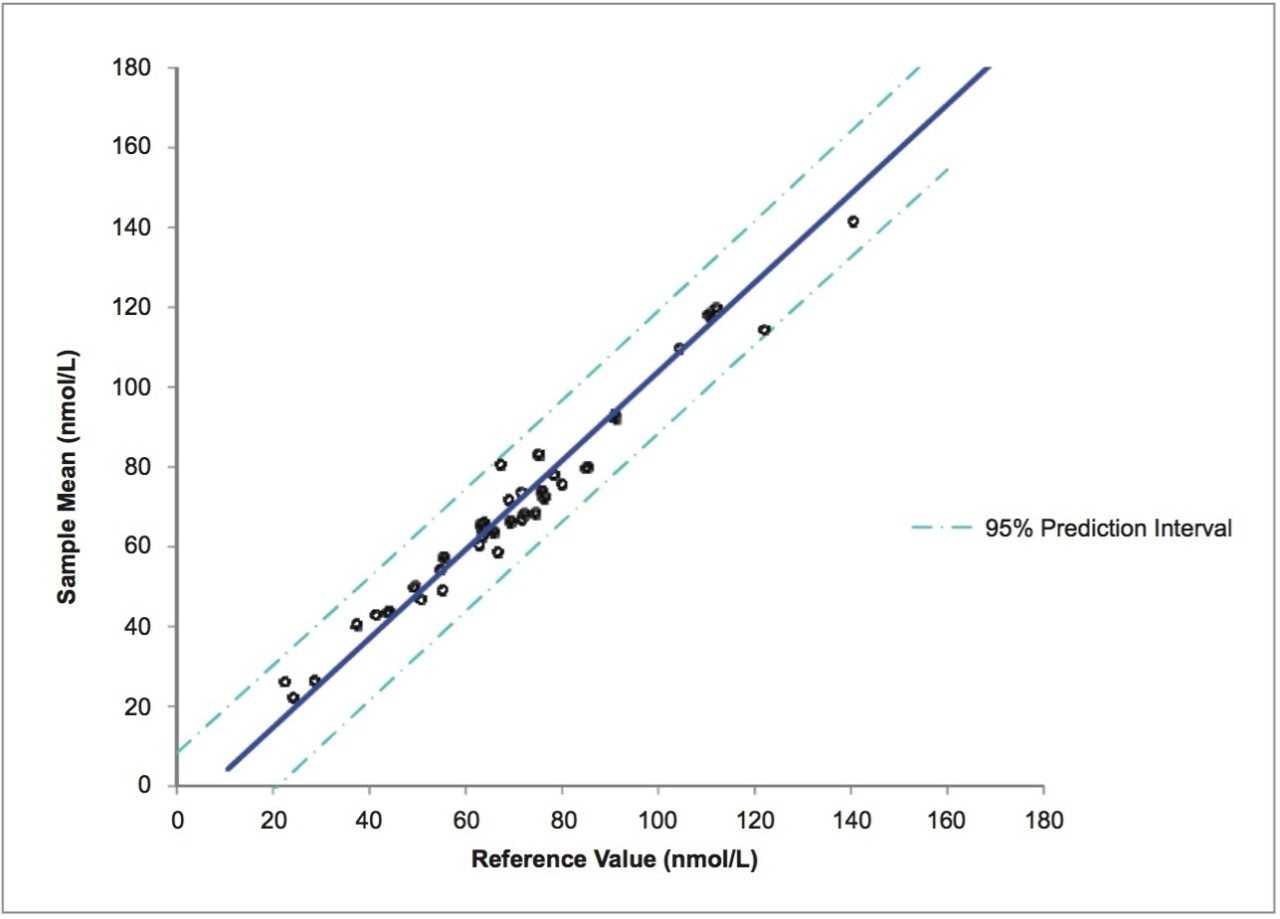
The MassTrak Vitamin D assay achieved a mean bias of 0.6% from the VDSCP reference values and a mean imprecision of 4.9%, meeting the certification performance criteria. Figure 3 shows the agreement between mean MassTrak Vitamin D measured concentrations (n=40) and the values assigned by the reference measurement procedure. The equation of the linear regression analysis was described as: MassTrak Vitamin D Assay = 1.1143(VDSCP) – 7.5584, with R2= 0.9715.
Metrological traceability of the MassTrak Vitamin D Kit calibrators and quality controls to NIST SRM2972 has been established, aiding laboratories in their compliance to ISO 15189. The assay’s accuracy and precision has been demonstrated through certification from the CDC Vitamin D Standardization Certification Program.
Intended Use: The Waters MassTrak Vitamin D Kit is for the quantitative determination of 25-hydroxyvitamin D3 and 25-hydroxyvitamin D2 , which in combination provide the total 25-hydroxyvitamin D in human plasma and serum using an automated liquid handling system and Waters ACQUITY UPLC I-Class/Xevo TQD IVD System. Results are to be used as an aid in the assessment of vitamin D sufficiency.
*For information on availability, please contact your local sales representative.
720005886, January 2017