
Phthalates, esters of phthalic acid, are often used as plasticizers for polymers such as polyvinylchloride. They are widely applicable in various products including personal care goods, cosmetics, paints, printing inks, detergents, coatings, and food packaging. These phthalates have been found to leach readily into the environment and food as they are not chemically bound to plastics. As such they are known to be ubiquitously present in our environment. Phthalates have been reported to show a variety of toxic effects related to reproduction in animal studies, which has resulted in these compounds being considered as endocrine disruptors. Screening food and beverages for phthalates contamination is required by many legislative bodies, although regulations vary from country to country in regards to acceptable daily tolerances and specific migration limits. In this application note phthalates were separated on a reversed-phase column within 11 minutes using UPLC coupled to a tandem quadrupole mass spectrometer. Limits of detection (LOD) and quantification (LOQ) of seven key phthalates (DEHP, BBP, DBP, DNOP, DEP, DMP, and DINP) in the sample are presented.
Phthalates, esters of phthalic acid, are often used as plasticizers for polymers such as polyvinylchloride. They are widely applicable in various products including personal care goods, cosmetics, paints, printing inks, detergents, coatings, and food packaging. These phthalates have been found to leach readily into the environment and food as they are not chemically bound to plastics. As such they are known to be ubiquitously present in our environment.
Phthalates have been reported to show a variety of toxic effects related to reproduction in animal studies, which has resulted in these compounds being considered as endocrine disruptors. Screening food and beverages for phthalates contamination is required by many legislative bodies, although regulations vary from country to country in regards to acceptable daily tolerances and specific migration limits.
Traditionally, phthalates have been analyzed by gas chromatography-mass spectrometry (GC-MS), where derivatization and/or extraction and sample preparation is often required to improve chromatographic separation.1 The resulting GC-EI-MS spectra can lack selectivity, where the base ion, used for identification and quantification of many common phthalates is the non-selective m/z 149 (C8H5O3) ion. Furthermore, background contamination of phthalates remains a significant challenge due to their ubiquitous presence.
In this application note phthalates were separated on a reversed-phase column within 11 minutes using UltraPerformance Liquid Chromatography (UPLC) coupled to a tandem quadrupole mass spectrometer. In order to assess the method’s applicability, various brands of distilled spirits were tested. Repeated injections of the samples were made to evaluate method robustness over a number of days. Limits of detection (LOD) and quantification (LOQ) of seven key phthalates (DEHP, BBP, DBP, DNOP, DEP, DMP, and DINP) in the sample will be presented.
|
LC system: |
ACQUITY UPLC H-Class |
|
Mobile phase A: |
Water + 0.1% formic acid |
|
Mobile phase B: |
Methanol + 0.1% formic acid |
|
Column: |
ACQUITY UPLC BEH C18, 1.7 μm, 2.1 x 50 mm |
|
Column temp.: |
40 °C |
|
Injection volume: |
10 μL |
|
Flow rate: |
0.5 mL/min |
|
Total run time: |
11.0 min |
|
Column: |
ACQUITY UPLC Isolator, 2.1 x 50 mm |
|
Needle wash: |
90% Methanol + 0.1 % formic acid |
|
Purge solvent: |
10% Methanol |
|
Seal wash: |
10% Methanol |
|
Time (min) |
%A |
%B |
Curve |
|---|---|---|---|
|
Initial |
60 |
40 |
6 |
|
0.50 |
60 |
40 |
6 |
|
5.00 |
1 |
99 |
6 |
|
8.00 |
1 |
99 |
6 |
|
8.50 |
60 |
40 |
6 |
|
11.00 |
60 |
40 |
6 |
|
MS System: |
Xevo TQD |
|
Ionization mode: |
ESI + |
|
Capillary voltage: |
0.5 kV |
|
Source temp.: |
150 °C |
|
Desolvation temp.: |
500 °C |
|
Desolvation gas: |
1000 L/hr |
|
Acquisition: |
Multiple Reaction Monitoring (MRM) |
Dimethyl phthalate (DMP), diethyl phthalate (DEP), dibutyl phthalate (DBP), benzyl butyl phthalate (BBP), bis(2-ethylhexyl) phthalate (DEHP), di-n-octyl phthalate (DNOP), and diiso-nonyl phthalate (DINP) were purchased from Sigma-Aldrich. The stock solutions for each phthalate were prepared at 1 mg/mL in methanol.
The samples analyzed in this application note consisted of an assortment of various distilled beverages purchased in the U.S.A. The sample description for each of the six samples is listed in Table 1.
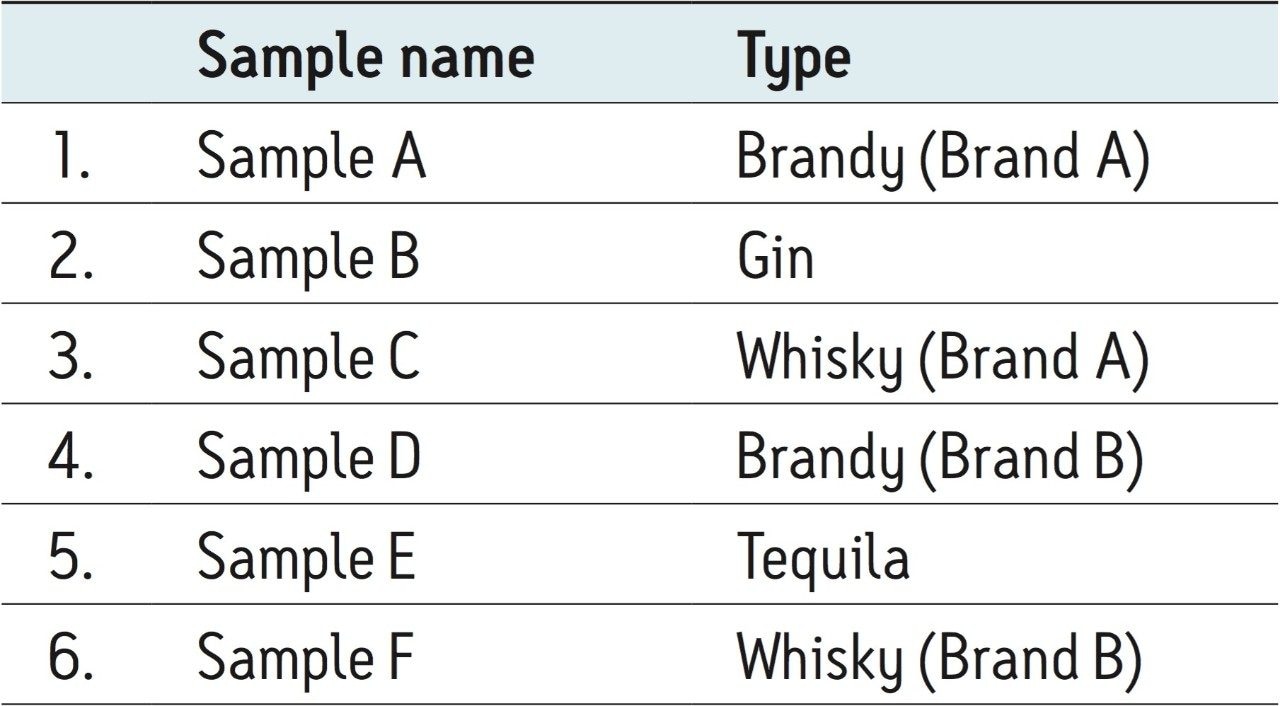
The distilled beverage samples were diluted 1:1 with water and placed in LCGC Certified Glass 12 x 32 mm Screw Neck Total Recovery Vials (p/n: 186000384C) for LC-MS/MS analysis.
For the matrix matched spiked calibration, Sample F was spiked with seven phthalates at 5, 10, 20, 50, and 100 ppb (µg/L) and diluted 1:1 with water. It is important to note that the water used for sample preparation should be screened for phthalates. Any background contamination from the water will add to any response in the sample and must be accounted for.
The data was acquired using MassLynx Software, and processed using TargetLynx Application Manager.
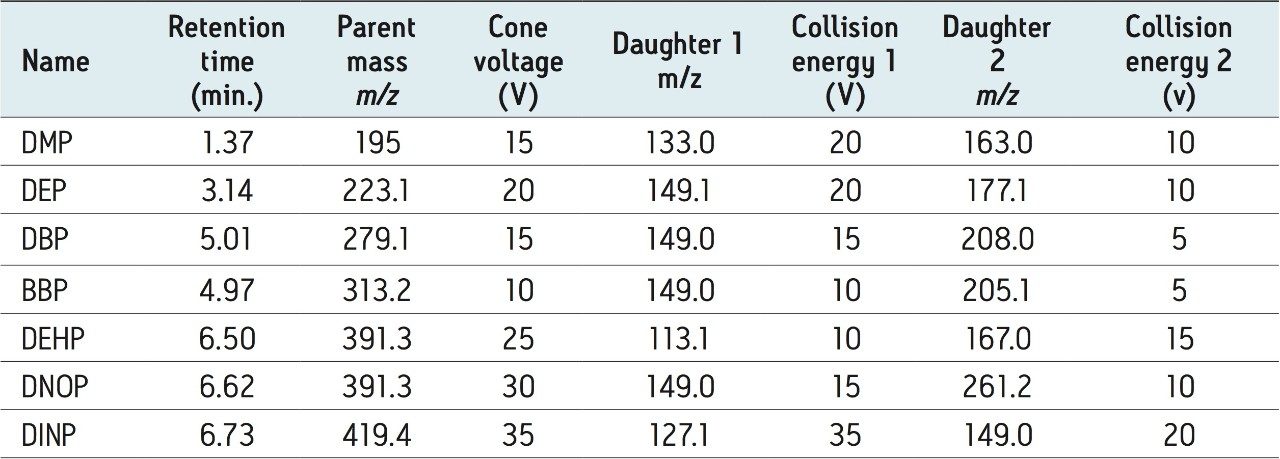
For all compounds, two MRM transitions were acquired for quantification and confirmation purposes. The MRM transitions, cone voltages, and collision energies for all compounds, along with expected retention times are shown in Table 2.
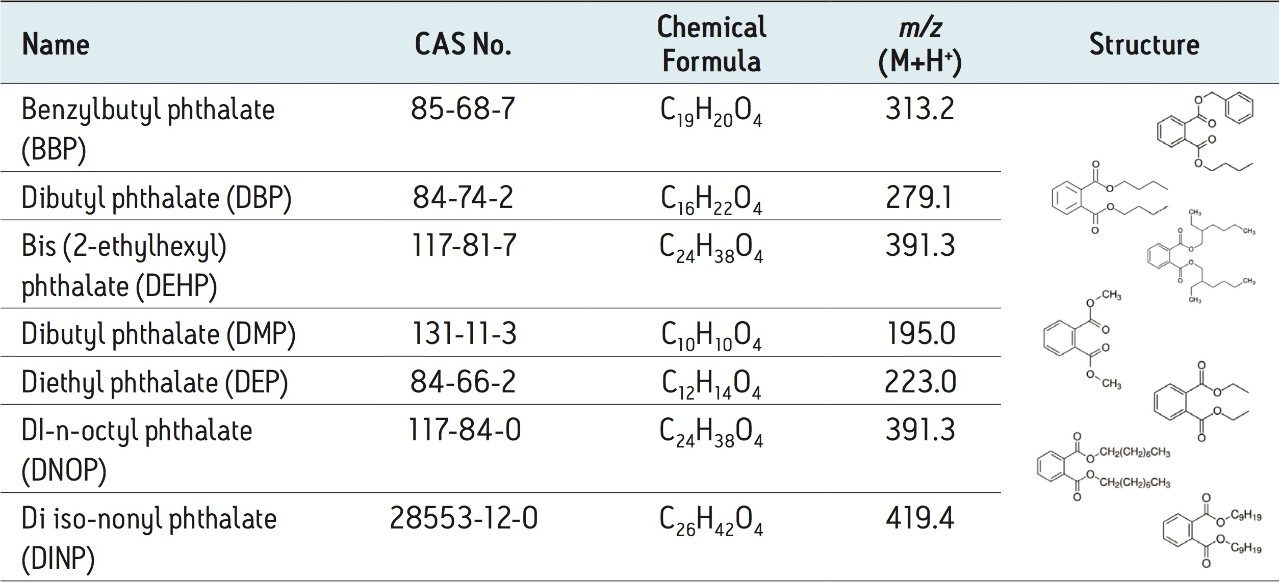
The chemical formula, mass (M+H+), and structure of all phthalates are shown in Table 3. The separation of the seven phthalates was achieved using the ACQUITY UPLC H-Class System in 11 minutes. The retention times of all phthalates are shown in Table 2.
Due to the ubiquitous contamination of phthalates in the environment, the background response from phthalates can interfere during sample analysis. In order to remove this background interference, an ACQUITY UPLC Isolator Column (p/n 186004476) with an extension tube that was placed in the flow path between the mobile phase mixer and the sample manager injector. Background phthalates are retained on the Isolator Column until the gradient elutes them through the analytical column. In this way, the phthalates from the sample elute earlier than the background phthalates. Hence the Isolator Column separates any background phthalate response from the phthalates in the sample to be analyzed. In addition, the peak shape of the phthalates from the background is much broader than those coming from the sample. Figure 1 shows an example of the separation of background phthalate contamination from the analyte of interest (BBP) in a solvent standard.
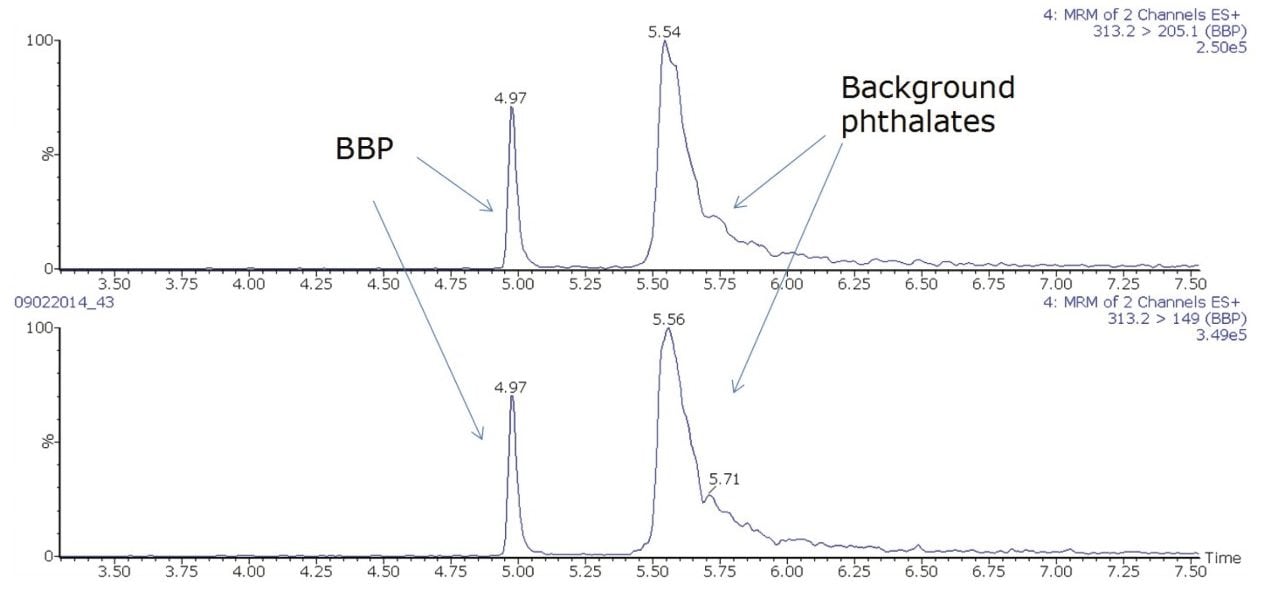
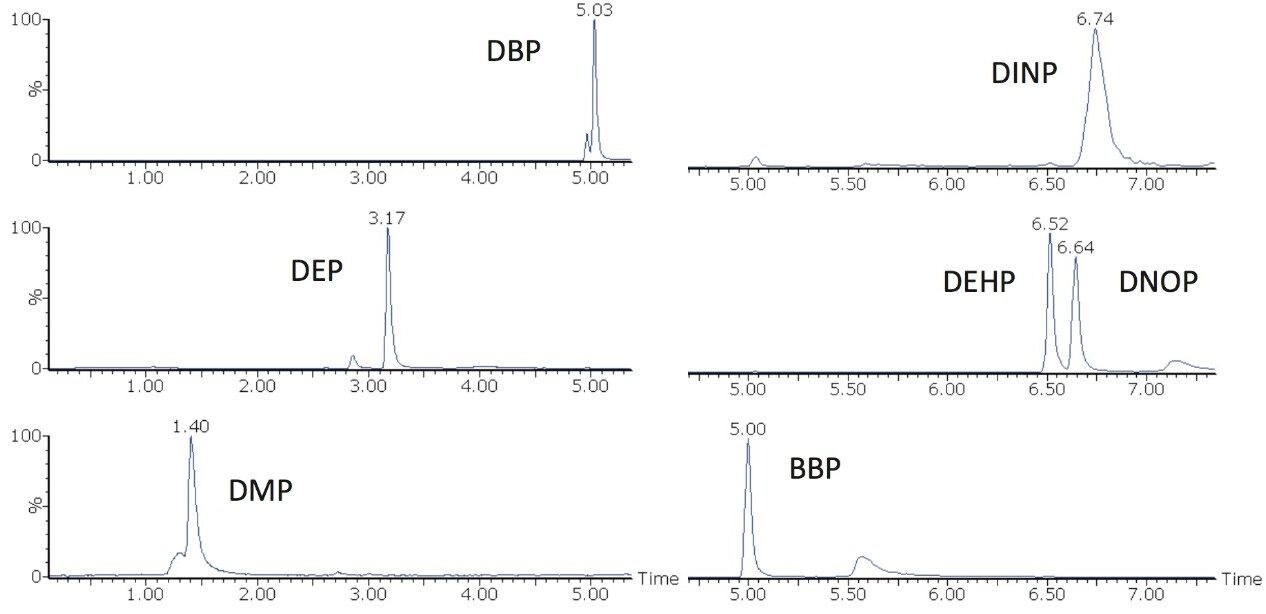
Figure 2 shows a chromatogram of each of the phthalates spiked in whisky sample F at 100 ppb (µg/L). All of the phthalates including the pair of isomers (DEHP and DNOP) were easily separated in less than 10 minutes. As discussed previously, the smaller background peaks in the chromatograms of DEP, DEHP/DNOP, and BBP are background contamination separated from the analytes of interest using the ACQUITY UPLC Isolator Column.
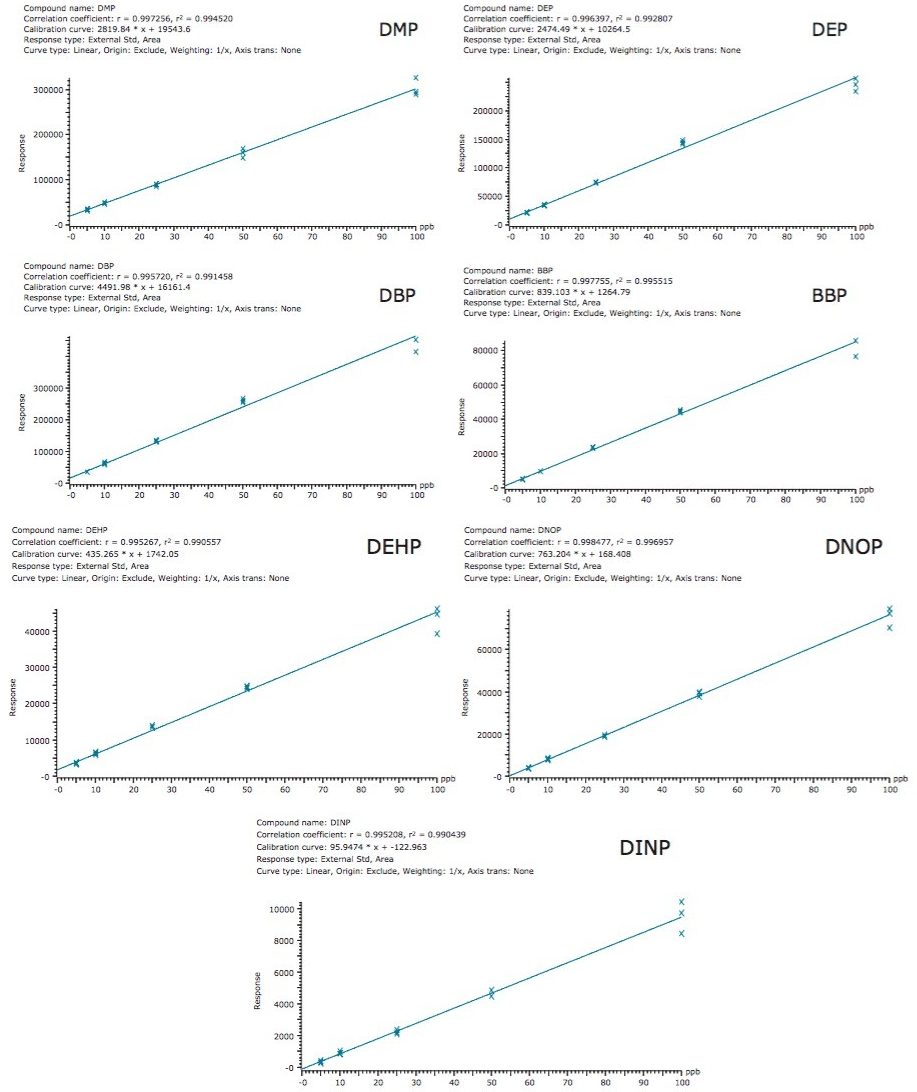
Sample F (whisky brand B) was chosen for the matrix spiked calibration curve. Sample F was pre-spiked with the seven phthalates at concentrations of 5, 10, 20, 50, and 100 ppb, then diluted 1:1 prior to analysis. Figure 3 shows the matrix spiked calibration curve for all of the phthalates in sample F. The co-efficient of determination (r2) of each calibration curve was >0.99 for all phthalates in sample F (Figure 3), and also in a solvent calibration curve (data not shown).
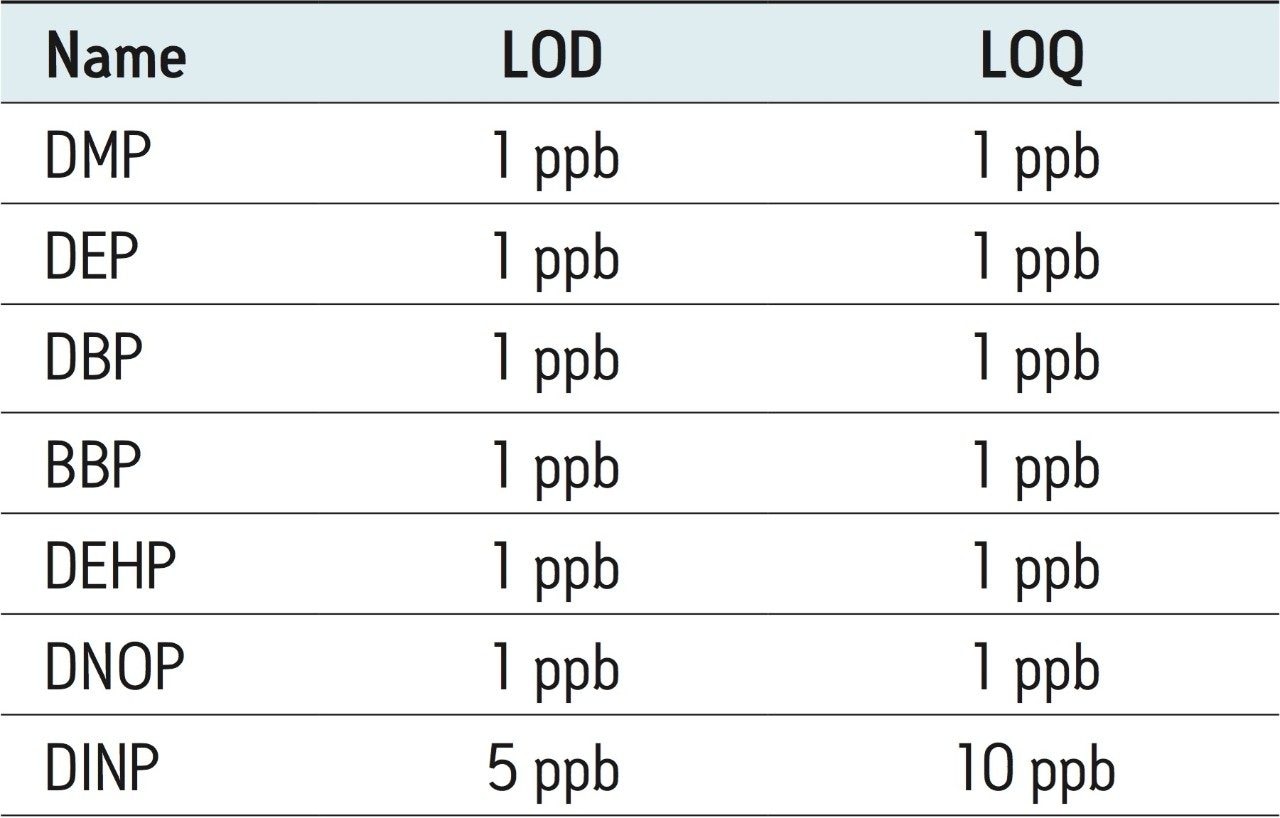
All phthalates were spiked into sample F at various levels (1 to 10 ppb) to determine LOD and LOQ. With the exception of DINP, all phthalates were easily detected at 1 ppb with a signal-to-noise (S/N) ratio >10. For DINP, the LOD and LOQ limits were based on the matrix spiked calibration standard that provided a signal-to-noise ratio >3 and >10, respectively. These are shown in Table 4 for sample F.
To study the applicability of the method to other distilled beverage types, all distilled beverage samples listed in Table 1 were spiked with the seven phthalates at 100 ppb (µg/L) and analyzed. All phthalates were successfully detected in all of the samples. Figure 4 shows the MRM chromatograms of BBP for each of the samples. BBP was successfully detected in all of the distilled beverage samples at 100 ppb.
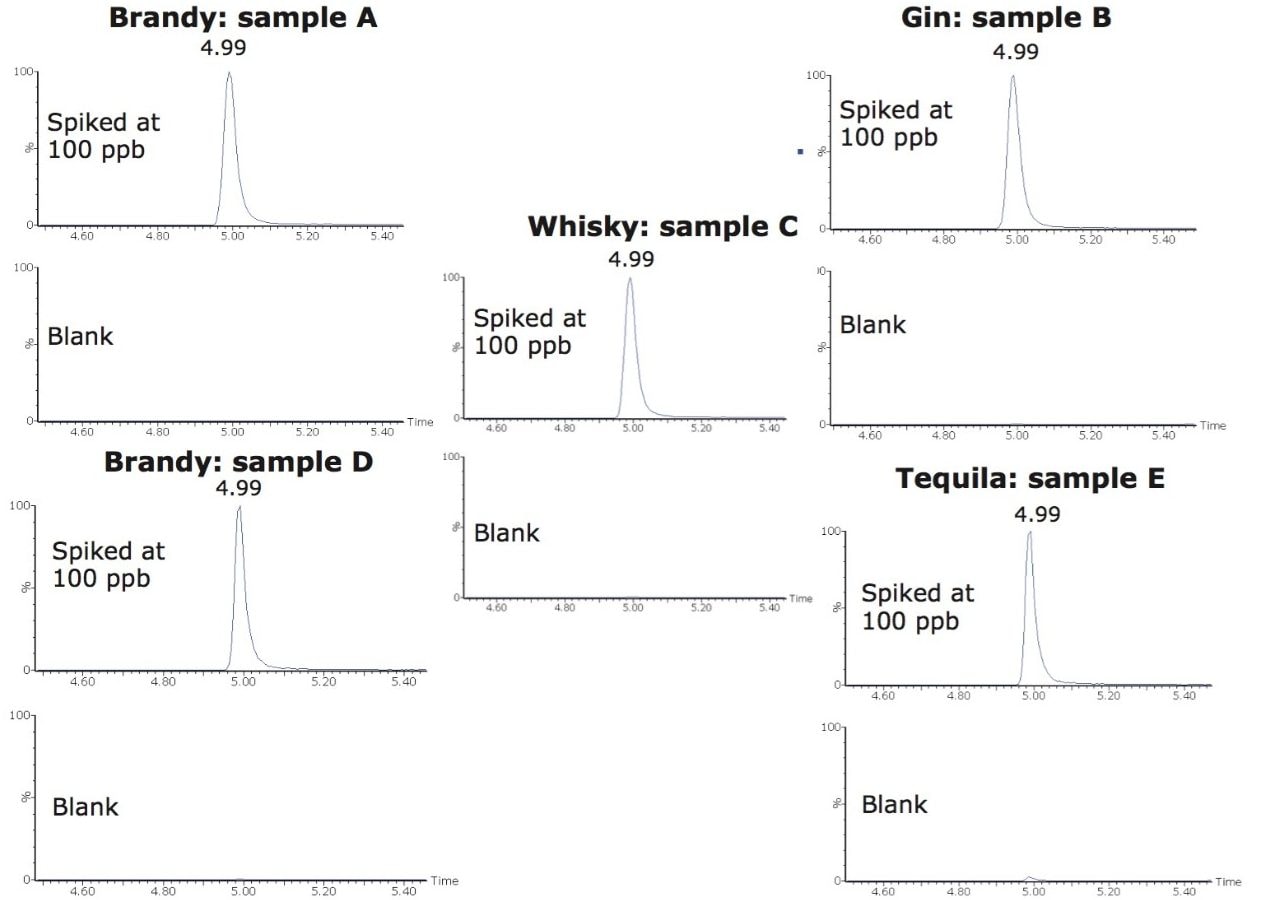
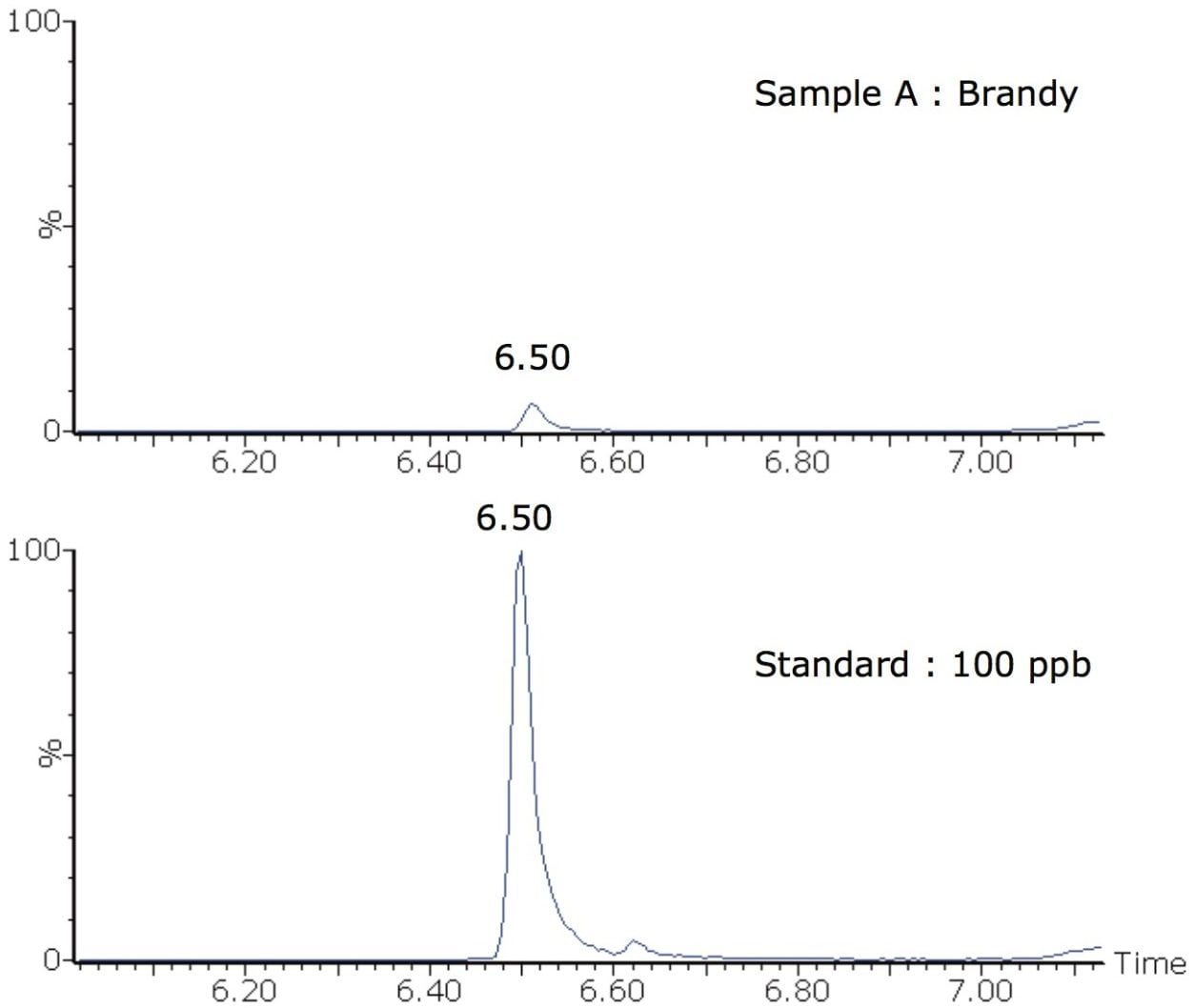
All of the alcoholic beverages (samples A to F) were analyzed in triplicate. For the majority of samples, no phthalates were detected. There was a trace level of DEHP detected in brandy A (<5 ppb). Figure 5 shows a chromatogram of DEHP (2 MRM transitions) in a solvent standard and in brandy A.
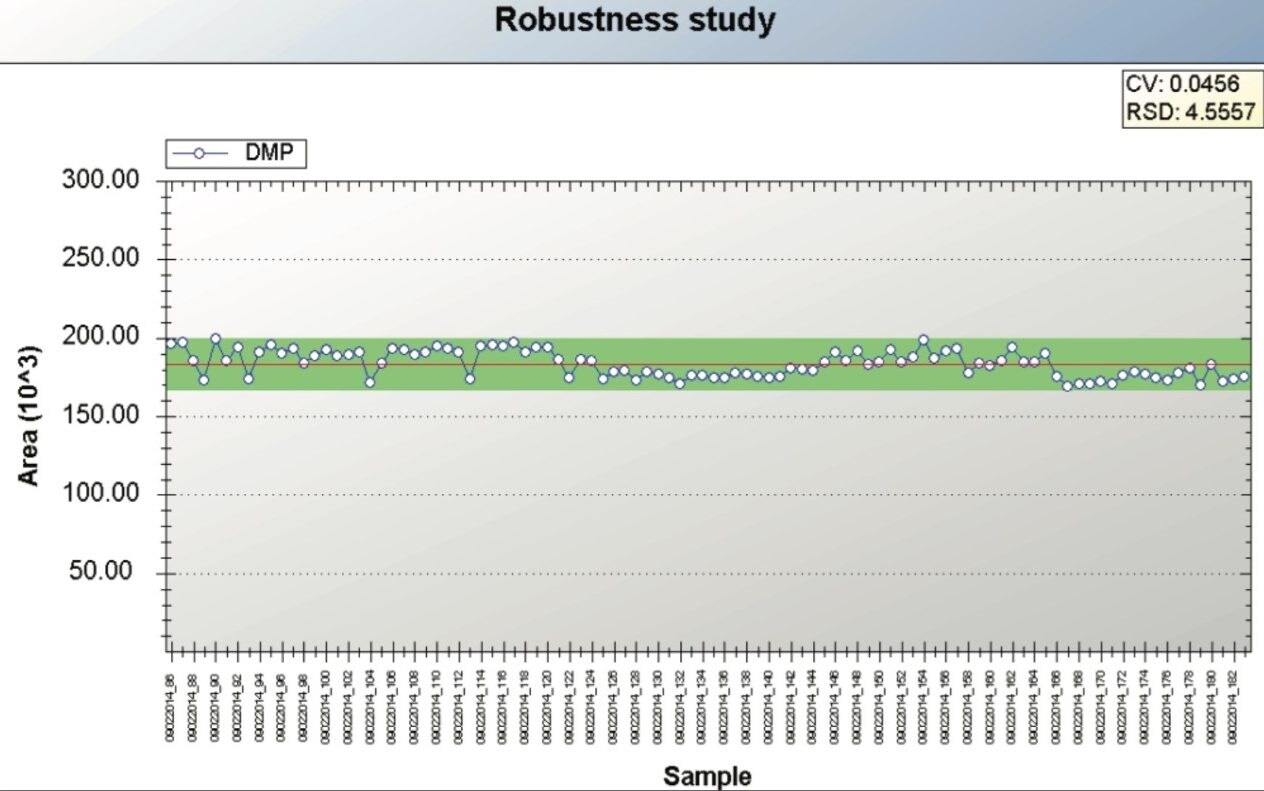
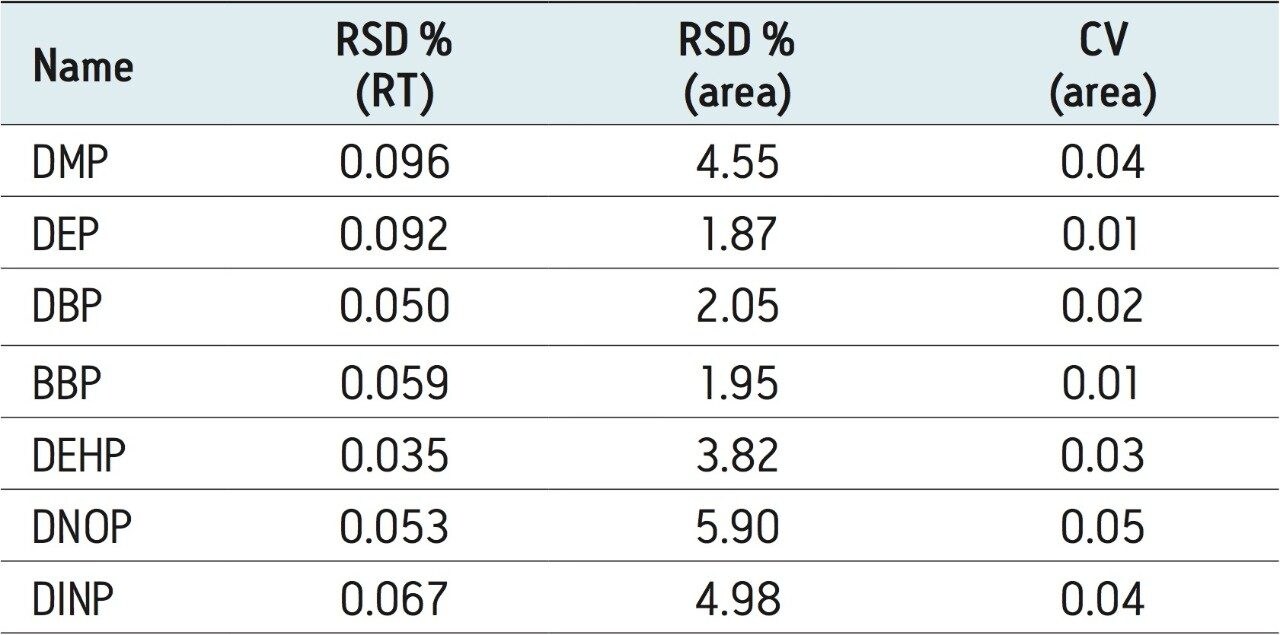
To assess the repeatability and robustness of the method, 100 injections of sample F spiked at 100 ppb were performed. Figure 6 shows an example TrendPlot of DMP over these 100 injections. The %RSD (retention time and area count) and relative precision (CV) for all compounds are shown in Table 5. The %RSDs for area count and retention time were <6% and 0.1% respectively for all compounds over the 100 injections.
The authors would like to acknowledge Robert Fotheringham and Edward Hayward at Chivas Brothers, Glen Keith Technical Centre, Glen Keith Distillery, Station Road, Keith, Banffshire, AB55 5BU, Scotland for their valuable input to this application.
720005403, May 2015