This is an Application Brief and does not contain a detailed Experimental section.
This application brief demonstrates the analysis of midazolam on Oasis MCX.
Midazolam is used to produce sleepiness or drowsiness and to relieve anxiety before surgery or certain procedures. In this example, the sample size was limited (less than 100 uL of plasma) and therefore the Oasis μElution plate was used.
Weak Base: pKa ~ 6.15 and therefore Oasis MCX was used.
|
Oasis MCX 96-Well μElution Plate |
|
|---|---|
|
Condition: |
200 μL MeOH |
|
Equilibrate: |
200 μL H2O |
|
Load: |
100 μL sample (50 μL plasma diluted 1:1 with 4% H3PO4) |
|
Wash 1: |
200 μL 2% HCOOH |
|
Wash 2: |
200 μL MeOH |
|
Elute: |
50 μL (25 μL x 2) 5% NH4OH in MeOH |
|
Dilute: |
100 μL H2O or 100 μL 2% FA in H2O to neutralize |
|
Inject: |
10 μL |
|
Column: |
SunFire C18 2.1 x 20 mm IS, 3.5 μm |
|
Mobile phase A: |
10 mM CH3COO-NH4+, pH 5.5 |
|
Mobile phase B: |
MeOH with 10 mM CH3COO-NH4+, pH 5.5 |
|
Flow rate: |
0.4 mL /min |
|
Instrument: |
2777 Sample Manager and 1525μ Binary HPLC Pump |
|
Time(min) |
Profile |
|
|
%A |
%B |
|
|
0.0 |
95 |
5 |
|
3.0 |
5 |
95 |
|
4.0 |
5 |
95 |
|
4.1 |
95 |
5 |
|
5.0 |
95 |
5 |
|
ESI- source temp: |
150 °C |
||
|
Desolvation temp: |
350 °C |
||
|
Cone gas flow: |
50 L /Hr |
||
|
Desolvation gas flow: |
600 L /Hr |
||
|
Collision cell: |
2.2e-3 bar (Ar gas) |
||
|
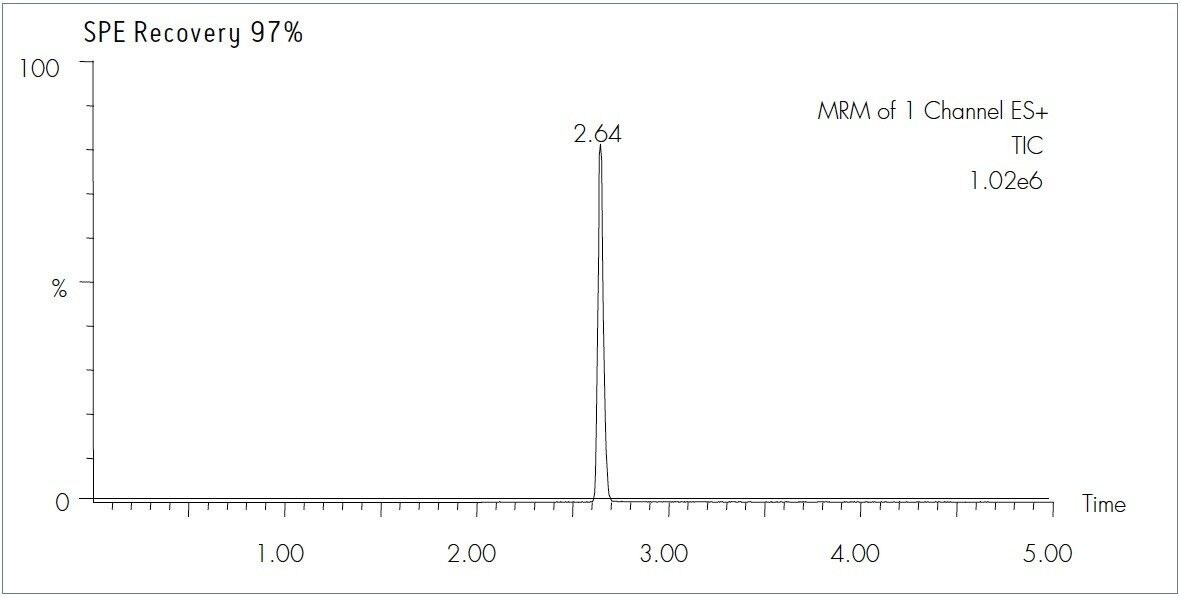
Midazolam |
MRM Transition |
Cone (V) |
CID (eV) |
|
m/z 326.2 → 291.2 |
40 |
28 |
WA60087, June 2007