
This application note describes a two and a half minute analysis of a blend of 10 polymer additives using the Waters ACQUITY UPLC System with PDA and ELS detection and Waters Empower 2 Software. With the built-in advanced mathematical algorithms, the polymer additives were quantitatively identified in a single run. The analysis is fast and reproducible. The ability to quickly and unambiguously analyze for polymer additives can facilitate workflow in quality control, new product development, deformulation of competitive polymer products, and product troubleshooting in the manufacturing of polymer additives as well as polymer and plastic products.
Enables rapid, sensitive, baseline resolved separations, and information rich data for a blend of polymer additives
Polymer additives protect and enhance the performance of polymer and plastic products throughout the cycle of manufacturing, processing, storage and final applications. Products used every day such as fibers, textiles, furniture, sports equipment, packaging, wire and cable, consumer electronics, telecommunication equipment, automobiles, and airplanes are all made entirely or partially of polymers and plastics. Their widespread use results not only from the development of new polymer chemistry and resins but also from the advancement of polymer additives. A variety of additives are used in polymer resin processing to generate products with specific processing characteristics and functional properties (color, shape, mechanical strength) as well as resistance to heat, flame, oxidation, aging, and light degradation.1-2
In manufacturing, a synergistic blend of polymer additives is incorporated into polymer and plastic products; small differences in the mixture can dramatically affect the characteristics of the products. To ensure that the intended amount of additive is in the polymer solution, accurate, reliable, and robust analytical methods are needed in QC and Central Analytical labs. Chromatographic techniques are the most widely used methods for the analysis of polymer additives. The typical separation time using conventional HPLC is approximately 20 to 40 minutes.2-6
This application note describes a two and a half minute analysis of a blend of 10 polymer additives using the Waters ACQUITY UPLC System with PDA and ELS detection and Waters Empower 2 Software. With the built-in advanced mathematical algorithms, the polymer additives were quantitatively identified in a single run. The analysis is fast and reproducible. The ability to quickly and unambiguously analyze for polymer additives can facilitate workflow in quality control, new product development, deformulation of competitive polymer products, and product troubleshooting in the manufacturing of polymer additives as well as polymer and plastic products.
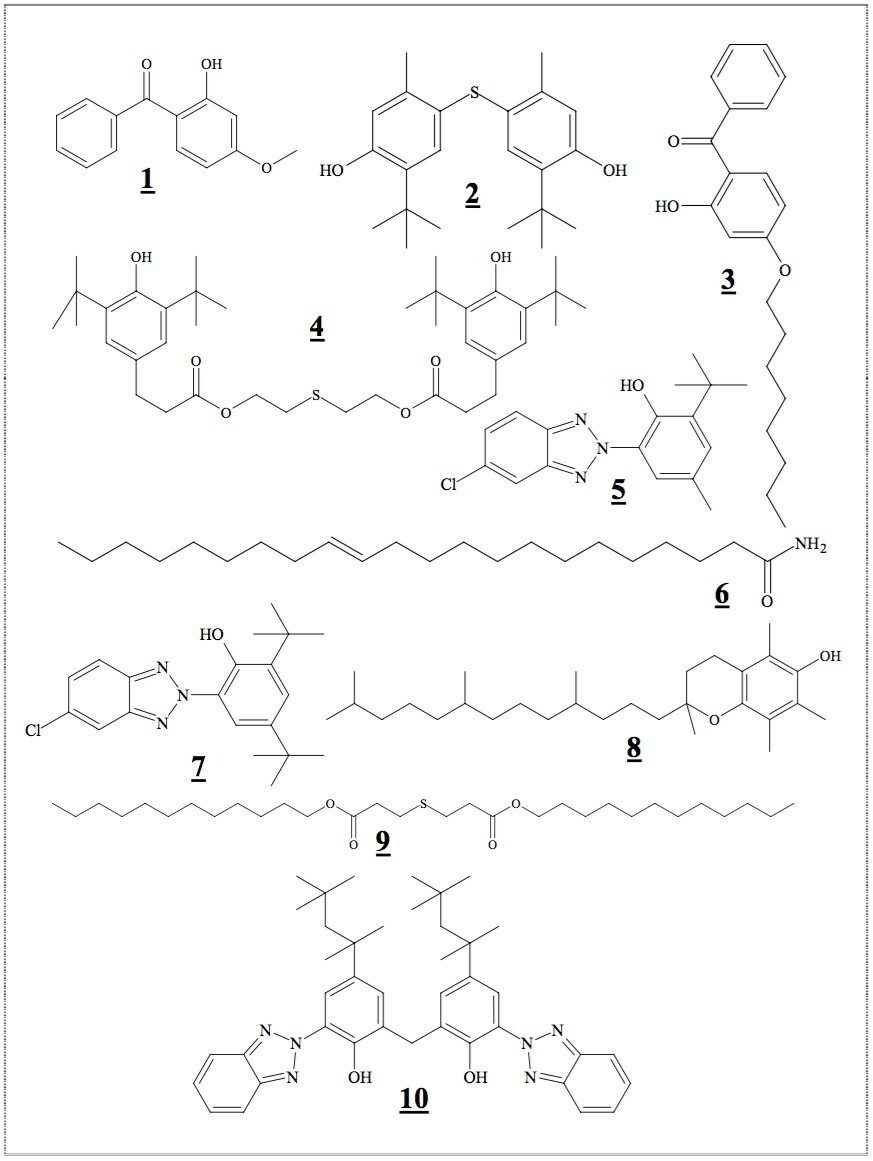
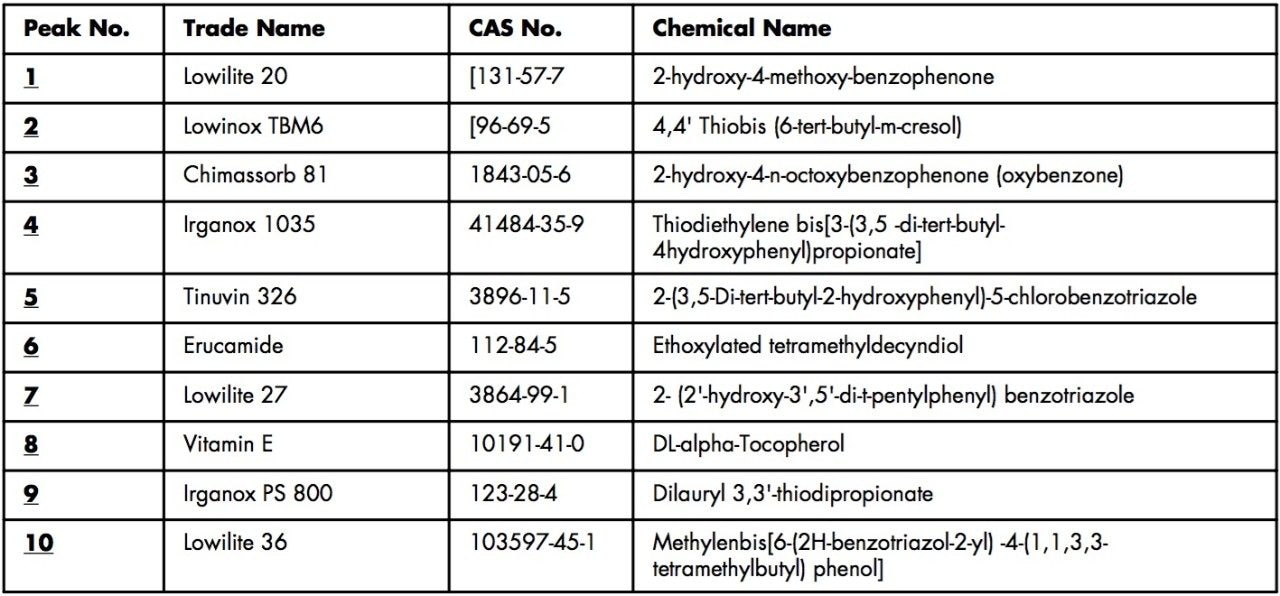
Analytes are Lowilite 20 (1), [131-57-7]; Lowinox TBM6 (2), [96-69-5]; Chimassorb 81 (3), [1843-05-6]; Irganox 1035 (4), [41484-35-9]; Tinuvin 326 (5), [3896-11-5]; Erucamide (6), [112-84-5]; Lowilite 27 (7), [3864-99-1]; Vitamin E (8), [10191-41-0]; Irganox PS 800 (9), [123-28-4]; and Lowilite 36 (10), [103597-45-1]. 1-4, and 8 were dissolved in CH3CN to make 2 mg/mL stock solution. 5 and 7 were dissolved in CH3CN/DMSO (1:1 by volume) to make 1 mg/mL stock solution. 6 was dissolved in acetone/DMSO (1:1 by volume) to make 1 mg/mL stock solution. 9 was dissolved in acetone to make 2 mg/mL stock solution. 10 was dissolved in CHCl3 to make 2 mg/mL stock solution. The stock solutions were mixed and diluted with CH3CN to give a working solution with 125 ppm of 1-10. Seven levels of calibration standards having 10, 15, 20, 25, 30, 35, and 40 ppm of 1-10 were prepared by dilution of the 125 ppm working solution with CH3CN.
|
System: |
ACQUITY UPLC w/PDA and ELS detectors |
|
Software: |
Empower 2 |
|
Weak wash: |
CH3CN (600 μL) |
|
Strong wash: |
CH3CN (600 μL) |
|
Seal wash: |
90:10 Water: CH3CN (5 min) |
|
Column temp.: |
50 °C |
|
Flow rate: |
1.0 mL/min |
|
Injection: |
2 μL (full loop) |
|
Detection: |
PDA 210 to 500 nm |
|
Sampling rate: |
20 pts/s |
|
Filter response: |
0.1 s |
|
Column: |
ACQUITY UPLC BEH C18 2.1x 50 mm |
|
Mobile phase A: |
0.05 v% of TFA in H2O |
|
Mobile phase B: |
0.05 v% of TFA in CH3CN |
|
Linear Gradient: |
50% to 100% B in 1.4 minutes, hold for 1.1 minutes at 100% B |
Note: Column equilibrated with 50%B for 2.5 min before each injection.
|
Gain: |
500 |
|
N2 Gas pressure: |
40 psi |
|
Drift tube temp.: |
57 °C |
|
Nebulizer: |
Cooler |
|
Date rate: |
20 pt/s |
|
Time constant: |
0.1 |
Figure 1 shows the chemical structures of polymer additives (1-10): light stabilizers and UV absorbers (1, 3, 5, 7, and 10), antioxidants and heat stabilizers (2, 4, 8, and 9), slip and mold release agent (6). They are commonly used to improve the performance of polymer and plastic products based on the following resins: ABS, PC, PE, PP, PVC, Acrylics, Polyacetal, Polyamides, Polyesters, Polystyrene, Polyurethanes, Elastomers, and Rubbers.
A blend of the polymer additives (1–10) was separated in 2.5 minutes using the Waters ACQUITY UPLC System with a linear gradient method.
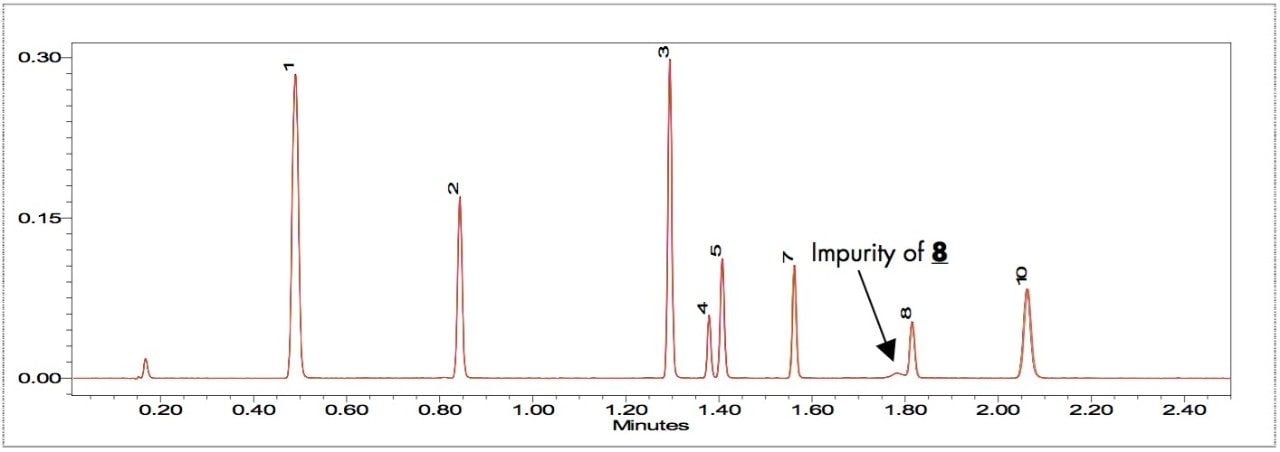
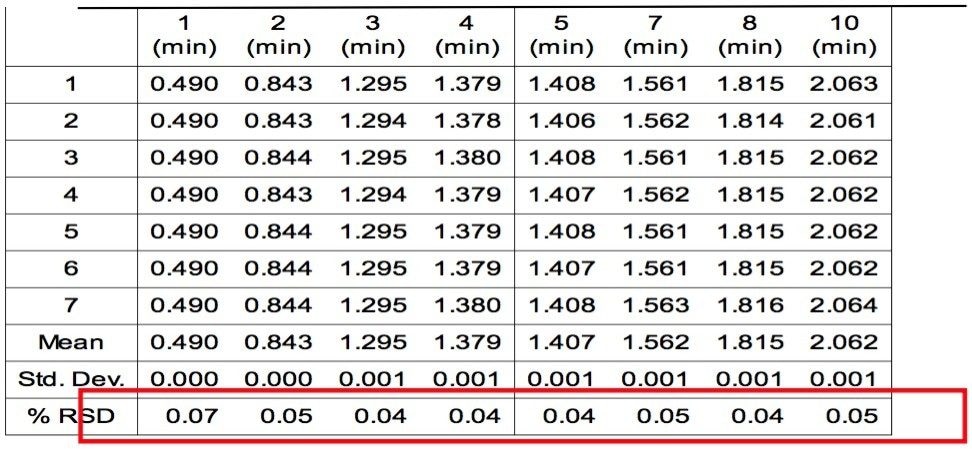
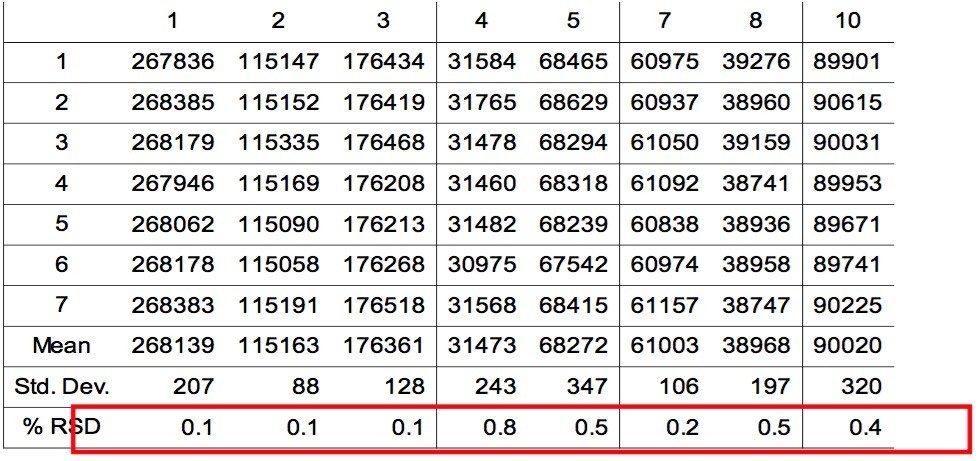
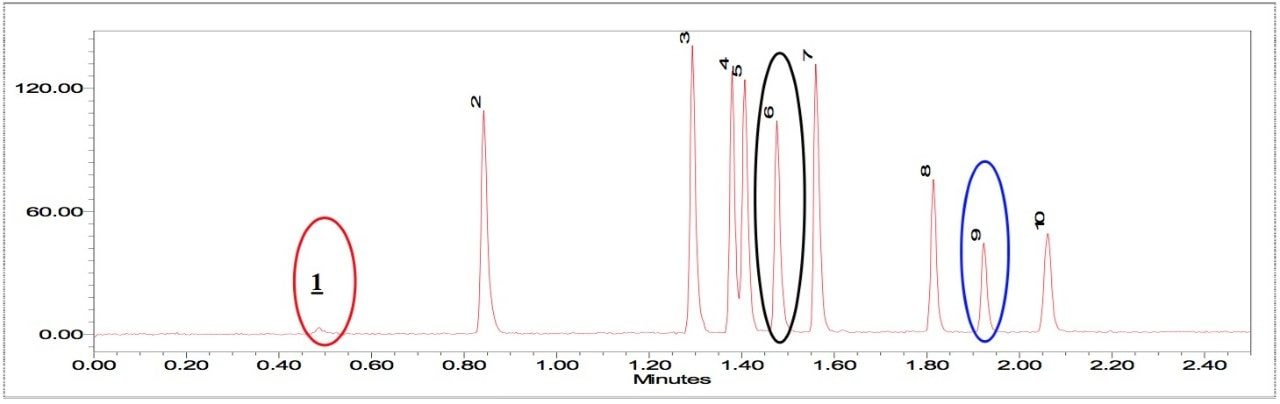
Figure 2 is an overlay of 7 replicate injections of PDA timed wavelength chromatograms. Visual examination shows the overall reproducibilty is excellent. The chromatograms show that eight polymer additives are well-resolved by the gradient method. The additives Erucamide and Irganox PS 800 (6 and 9) don't have a strong UV chromophore and weren't observed. An unknown impurity in the Vitamin E (8) sample was found. Tables 1 and 2 show the retention times and peak area of each polymer additive observed in the 7 replicate injections with statistical analysis results generated by Empower. The excellent %RSD results are good indicators of the robustness and suitability of UPLC with BEH column chemistry for the analysis of polymer additives.
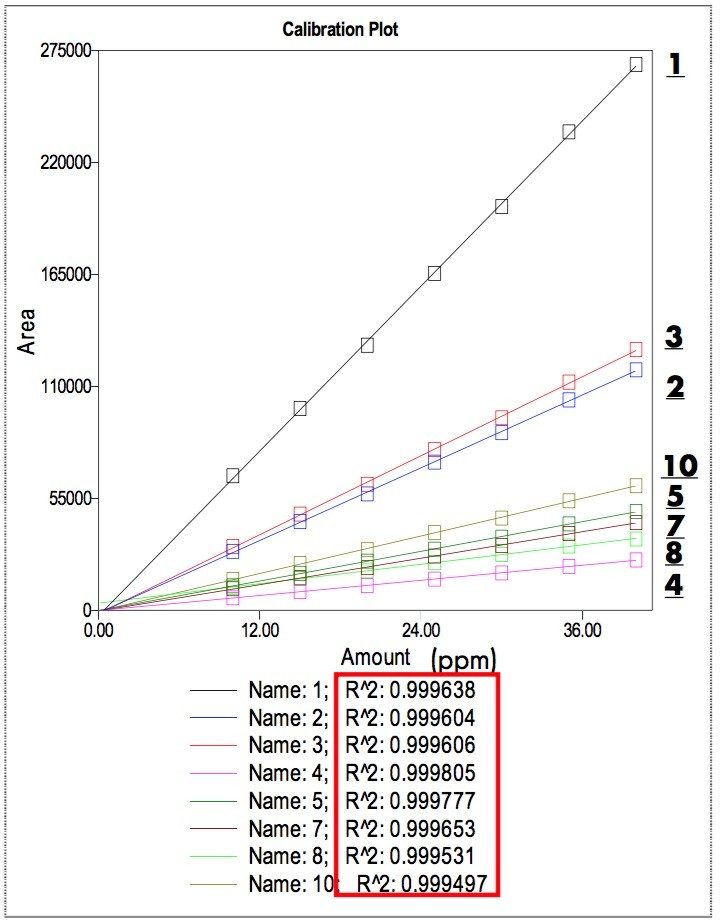
Seven levels of calibration standards (10 to 40 ppm) were analyzed. With Empower’s built-in advanced mathematical features, calibration curves were created from the standards and the quantities of analyte in each sample were calculated automatically. Figure 3 shows the calibration plots generated by Empower, using the peak areas vs the concentration. The linearity of the calibration curves was excellent with the R2 values (residual sum of squares) above 0.9995. Table 3 shows a typical analysis results for peak identification and quantification using a blend of polymer additives as a sample. The last column shows all the results matching well with actual value (10 ppm). The data suggest that the UPLC system is well suited for the quantitative analysis of polymer additives in sub-ppm.
UV photodiode array (PDA) detection combined with Empower 2 Software enables a powerful range of detection and identity confirmation possibilities for chromatographic separations. Empower 2 provides the capability of creating a PDA library from pure component peaks in user chromatograms. The library matching and peak purity features can be used to confirm peak identities and to give added confidence that spectrally distinct peaks are not-coeluting. Using Spectral Contrast theory, Empower 2 quantitatively compares the shapes of UV spectra during library matching and Peak Purity analysis.7-9 Figure 4 shows UV spectra, extracted from PDA chromatograms of polymer additive standards, that were used to create a library with names and retention times.
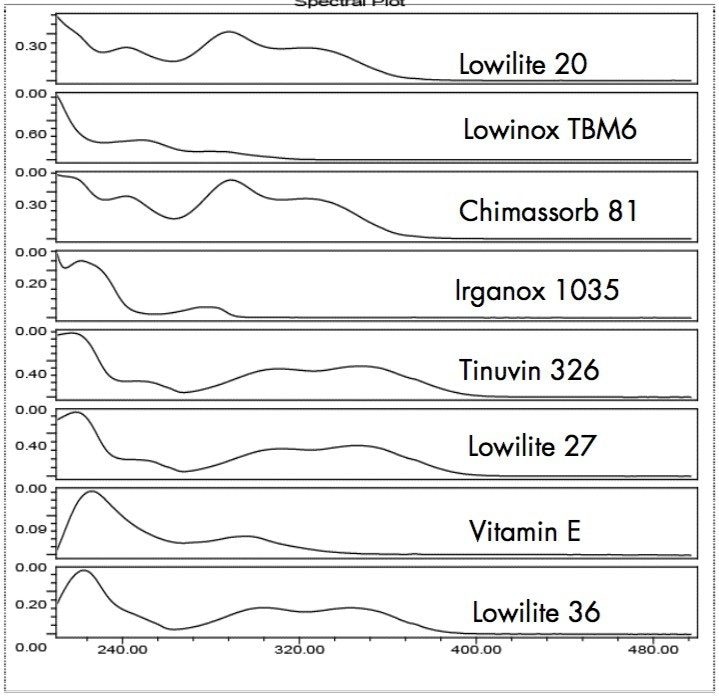
Table 3 is an example of a default Empower Report table with PDA library matching and Peak Purity results. In general, if the value of Match Angle is smaller than Match Threshold and the value of Purity Angle is smaller than Purity Threshold, the results indicate that the analyte is well separated and well matched with the PDA library standard.
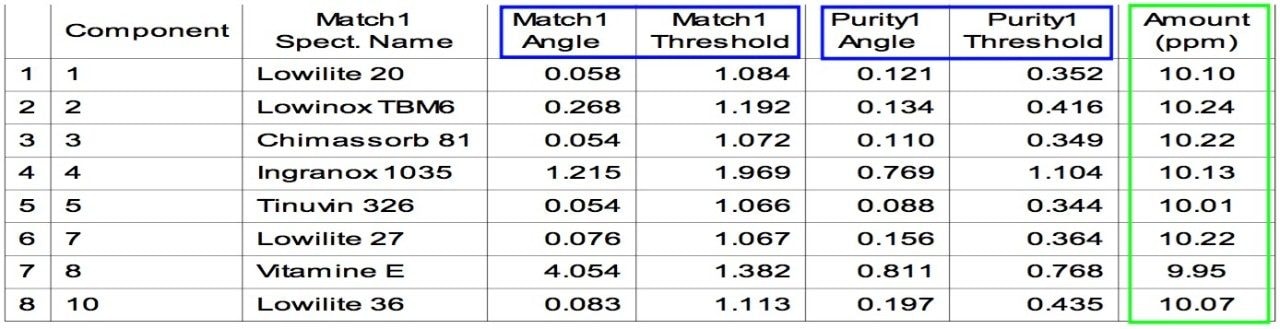
The data in Table 3 indicate that all the UV absorbing polymer additives except 8 were well separated and matched with PDA libraries. The values obtained from Empower indicate that peak 8 is not spectrally pure, perhaps suggesting coeuluting components. As indicated earlier in Figure 2 there is an overlap between the peaks of 8 and its impurity. Empower indicated this by returning a Purity1Angle greater than a Purity1Threshold.
To further characterize the impurity of 8, a longer BEH C18 column (2.1 x 100 mm) might be used to optimize the separation and a mass spectrometer added as a detector. These experiments are outside the scope of this application note.
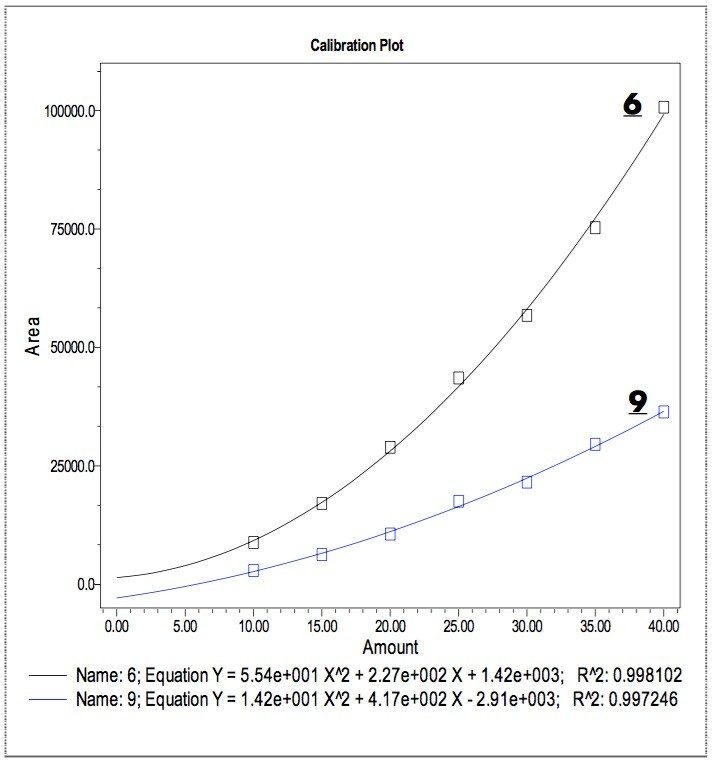
Figure 5 is an evaporative light scattering (ELS) chromatogram of a sample containing 40 ppm each of the additives (1-10). The chromatogram shows that nine (2-10) of the ten additives have significant response under the ELS detection conditions; the two non-UV absorbing additives (6 & 9) are well separated from the others. The low ELS response of 1 is could be related to its volatility.
The ELS data fit well with quadratic equations. Figure 6 shows the typical quadratic calibration plots generated by Empower, using the peak areas vs the concentration of analyte. The R2 values (residual sum of squares) for 6 and 9 are 0.998 and 0.997, respectively.
These results demonstrate the utility of combined PDA and ELS detectors with an ACQUITY UPLC System for analyzing polymer additives. With a single chromatographic run, all the UV and non-UV absorbing polymer additives can be analyzed simultaneously. Since many polymer additives lack a UV chromophore, an ELS detector, in conjunction with a PDA detector, is well suited for this type of analysis.
The Waters ACQUITY UPLC with PDA and ELS detectors is an ideal system for the analysis of polymer additives. It enables rapid, sensitive, baseline resolved separations, and information rich data for a blend of polymer additives. All analytes cannot be detected using a single detection technique. By employing complementary detectors, more information per chromatographic run can be obtained, thus dramatically increasing productivity. With Empower, the data obtained from both PDA and ELS detectors can be analyzed simultaneously for polymer additive quantification. The PDA library matching and peak purity functions can be automated to add confidence in peak identification for UV absorbing analytes. The easy to employ experimental conditions are suitable for R&D, analytical and service laboratories involved in polymer/plastics development and production. Additional applications of this methodology may include the evaluation of food and medicine contamination by polymer additives that migrate from packaging, medical tubing, and medical devices.
720001881, September 2006