For research use only. Not for use in diagnostic procedures.
This application note demonstrates the capabilities of the sample preparation and UPLC-MS/MS system to quantify 18 antiepileptic drugs and metabolites in plasma.
Pharmacokinetic interactions between antiepileptic drugs are a known phenomenon, therefore an accurate quantitative method may play a role in researching the pharmacokinetic and pharmacodynamic effects of administration of antiepileptic drugs.
Here we describe a clinical research method using protein precipitation of a plasma sample with internal standards. Chromatographic elution was completed within five minutes using a Waters CORTECS C8 Column on an ACQUITY UPLC I-Class System followed by detection on a Xevo TQD Triple Quadrupole Mass Spectrometer utilizing polarity switching (Figure 1).

Plasma calibrators and quality control materials were prepared in-house using pooled human plasma supplied by BioIVT (West Sussex, UK). Concentrated stock solutions were prepared from certified powders and solutions supplied by Cambridge Bioscience (Cambridgeshire, UK), Fisher (Loughborough, UK), Sigma-Aldrich (Dorset, UK), and Toronto Research Chemicals (Ontario, Canada). Stable labelled internal standards were supplied by Cambridge Bioscience (Cambridgeshire, UK), Sigma-Aldrich (Dorset, UK), and Toronto Research Chemicals (Ontario, Canada). The calibration range was 1–100 μg/mL for all analytes, except for oxcarbazepine, perampanel, and pregabalin (0.1–10 μg/mL), tiagabine (0.01–1 μg/mL), and valproic acid (2–200 μg/mL). In-house quality control samples were prepared in plasma at low, medium, and high concentrations of 2.5, 7.5, and 40 μg/mL for 10,11-dihydro-10-hydroxycarbamazepine, carbamazepine, felbamate, gabapentin, lacosamide, lamotrigine, levetiracetam, phenobarbital, phenytoin, primidone, topiramate, and zonisamide; 0.25, 0.75, and 4 μg/mL for oxcarbazepine, perampanel, pregabalin, and retigabine; 0.025, 0.075, and 0.4 μg/mL for tiagabine; and 5, 15, and 80 μg/mL for valproic acid.
To 50 μL of sample, 200 μL of internal standard in methanol was added, containing 5 μg/mL of valproic acid-2H6, 2.5 μg/mL of carbamazepine-2H2 15N, 10,11-dihydro-10- hydroxycarbamazepine-13C6, felbamate-2H4, gabapentin-2H4, lacosamide-2H6, levetiracetam-2H3, phenobarbital-2H5, phenytoin-2H10, primidone-2H5, topiramate-13C6 and zonisamide-13C2 15N, 0.625 of μg/mL lamotrigine-13C3 and oxcarbazepine-2H4, 0.5 μg/mL of perampanel-2H5, 0.25 μg/mL of pregabalin-13C3 and retigabine-2H4, and 0.03125 μg/mL of tiagabine-2H6. Tubes were placed on a multi-tube vortex mixer at 2500 rpm for 30 seconds, then centrifuged for two minutes at 16,100 g. Fifty microliters (50 μL) of supernatant were transferred to a 1-mL, 96-well plate and 350 μL water added. The plate was then centrifuged at 4,696 g for two minutes prior to analysis.
|
System: |
ACQUITY UPLC I-Class with Flow-Through Needle (FTN) |
|
Needle: |
30 μL |
|
Column: |
CORTECS C8 Column; 2.7 μm, 2.1 × 50 mm (p/n: 186008349) |
|
Mobile phase A: |
Water + 2 mM ammonium acetate |
|
Mobile phase B: |
Methanol + 2 mM ammonium acetate |
|
Needle wash solvent: |
80% aqueous methanol + 0.1% formic acid |
|
Purge solvent: |
5% aqueous methanol |
|
Seal wash: |
20% aqueous methanol |
|
Column temp.: |
50 °C (precolumn heater active) |
|
Injection volume: |
20 μL |
|
Flow rate: |
0.50 mL/min |
|
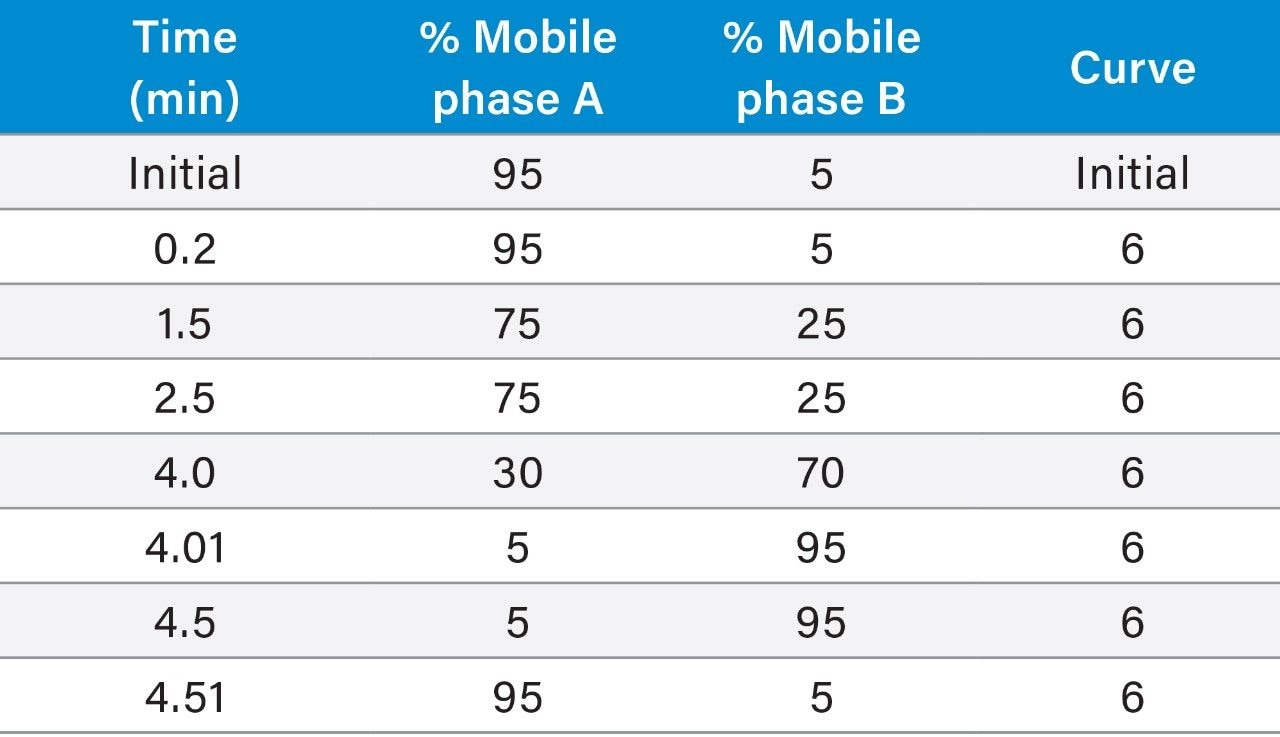
Gradient elution: |
(see Table 1) |
|
Run time: |
5.0 minutes (5.7 minutes injection-to-injection) |
|
System: |
Xevo TQD |
|
Resolution: |
MS1 (0.7 FWHM), MS2 (0.7 FWHM) |
|
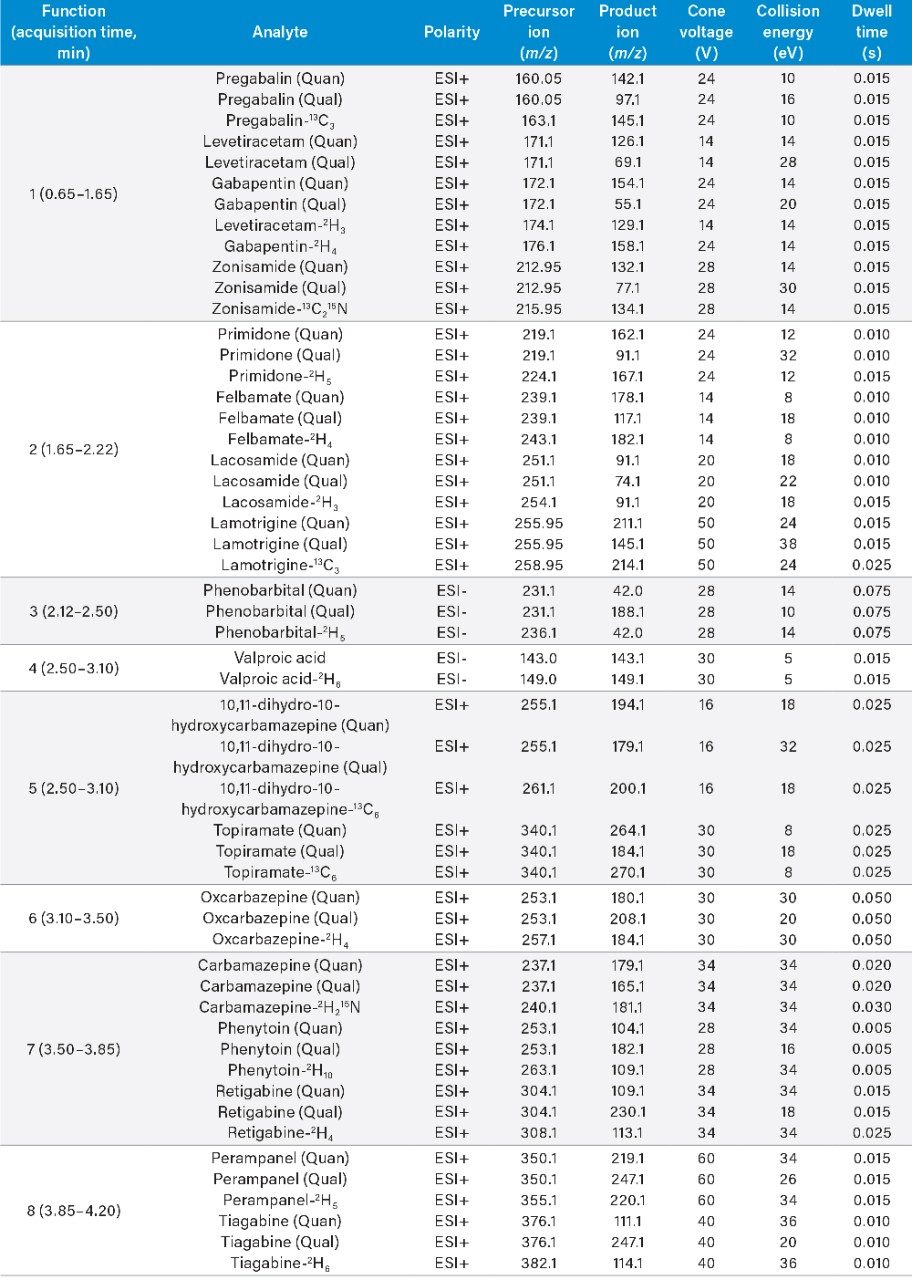
Acquisition mode: |
Multiple reaction monitoring (MRM) (see Table 2 for details) |
|
Polarity: |
ESI positive ionization/ESI negative ionization (ESI+/ESI-) |
|
Capillary: |
3.5 kV (ESI+)/0.8 (ESI-) |
|
Source temp.: |
150 °C |
|
Desolvation temp.: |
500 °C |
|
Cone gas: |
100 L/hr |
|
Inter-scan delay: |
0.003 seconds |
|
Polarity/mode switch inter-scan delay: |
0.020 seconds |
|
Inter-channel delay: |
0.003 seconds |
MassLynx Software v4.2 with TargetLynx Application Manager
No system carryover was observed following analysis of plasma samples with 100 μg/mL of 10,11-dihydro-10-hydroxycarbamazepine, carbamazepine, felbamate, gabapentin, lacosamide, lamotrigine, levetiracetam, phenobarbital, phenytoin, primidone, topiramate, and zonisamide; 10 μg/mL of oxcarbazepine, perampanel, pregabalin, and retigabine; 1 μg/mL of tiagabine; and 200 μg/mL of valproic acid.
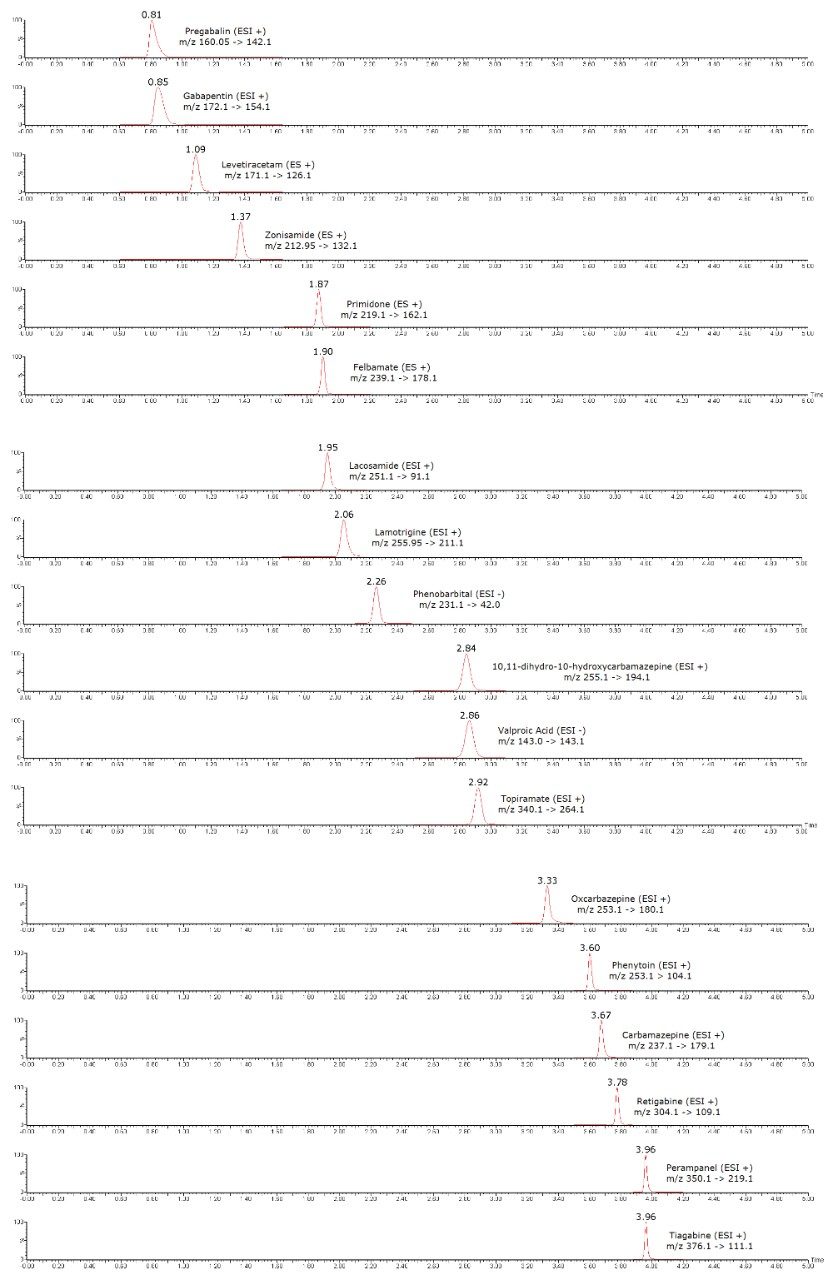
Figure 2 shows an example chromatogram for the analysis of the 18 antiepileptic drugs and metabolites.
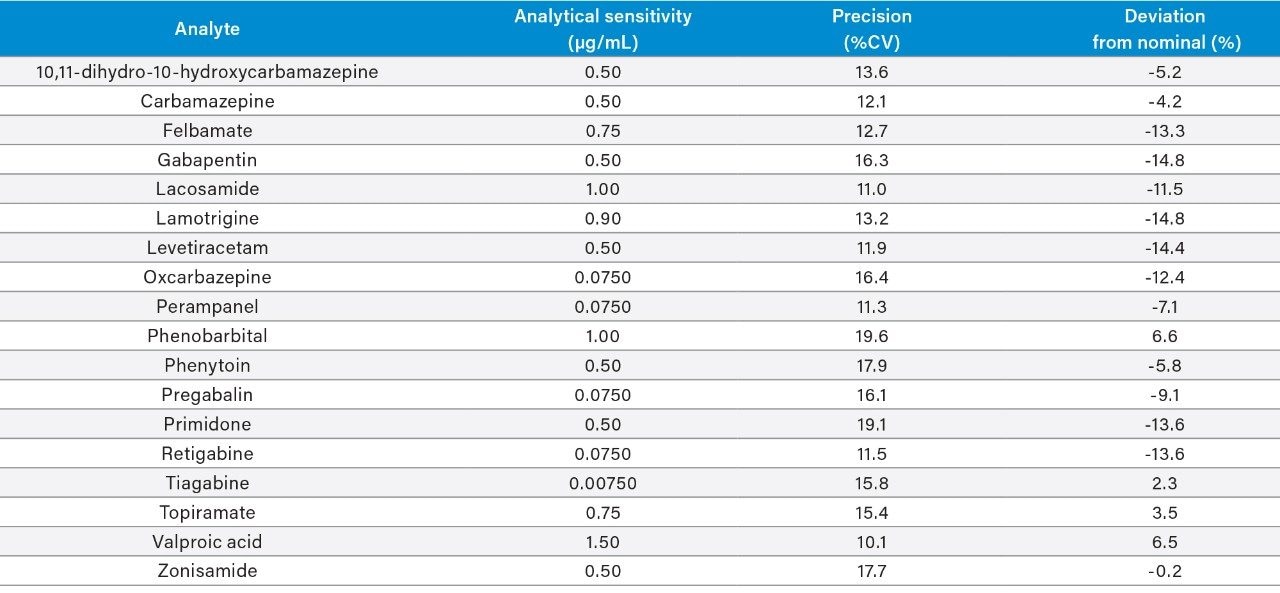
Analytical sensitivity was assessed by extracting and quantifying 10 replicates of low concentration samples prepared in plasma over five days (n=50). Investigations indicated the method would allow for precise quantification (≤20% CV, ≤15% bias) at the concentrations shown in Table 3.
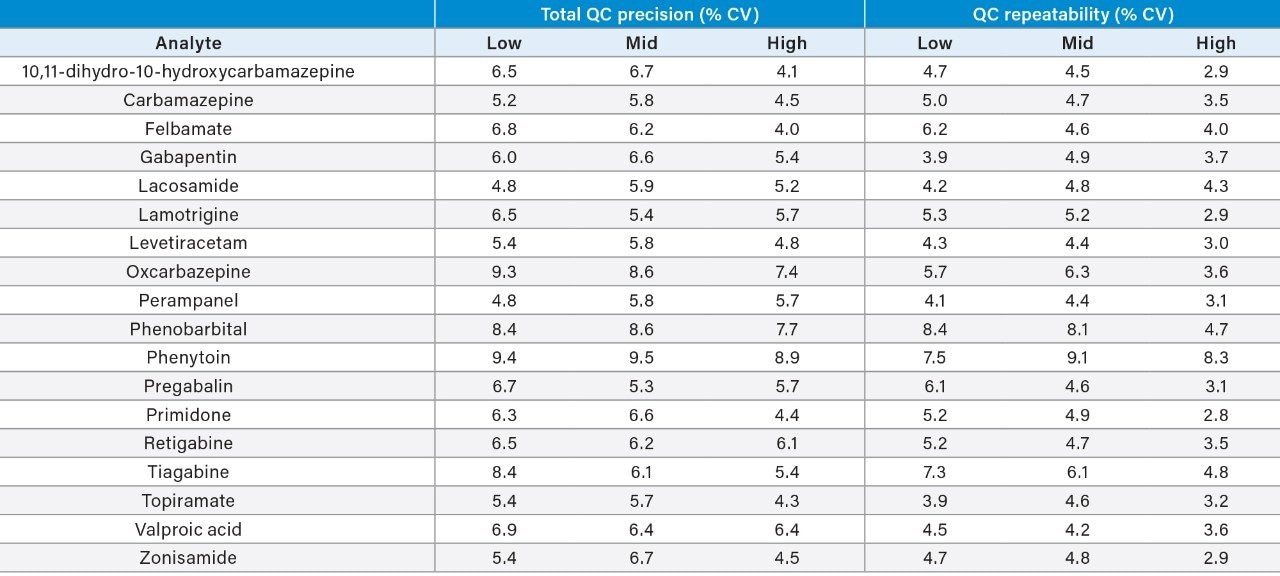
Total precision was determined by extracting and quantifying five replicates of three concentrations of plasma pools over five separate days (n=25). Repeatability was assessed by analyzing five replicates at each QC level. Table 4 presents results of these experiments, where total precision and repeatability at the three concentrations assessed was ≤9.5% RSD.
The method was shown to be linear over the range of 0.752–130 μg/mL for phenobarbital, topiramate, and zonisamide, when low and high pools were mixed in known ratios over the range. Linear fits were used to construct calibration lines. Carbamazepine, 10,11-dihydro-10-hydroxycarbamazepine, felbamate, gabapentin, lacosamide, lamotrigine, levetiracetam, phenytoin, and primidone were determined to have quadratic fits over 0.752–130 μg/mL. Similarly, oxcarbazepine, perampanel, pregabalin, and retigabine were quadratic over 0.0752–13.0 μg/mL; tiagabine over 0.00752–1.30 μg/mL; and valproic acid over 1.5–260 μg/mL. Quadratic fits were used to construct calibration lines.
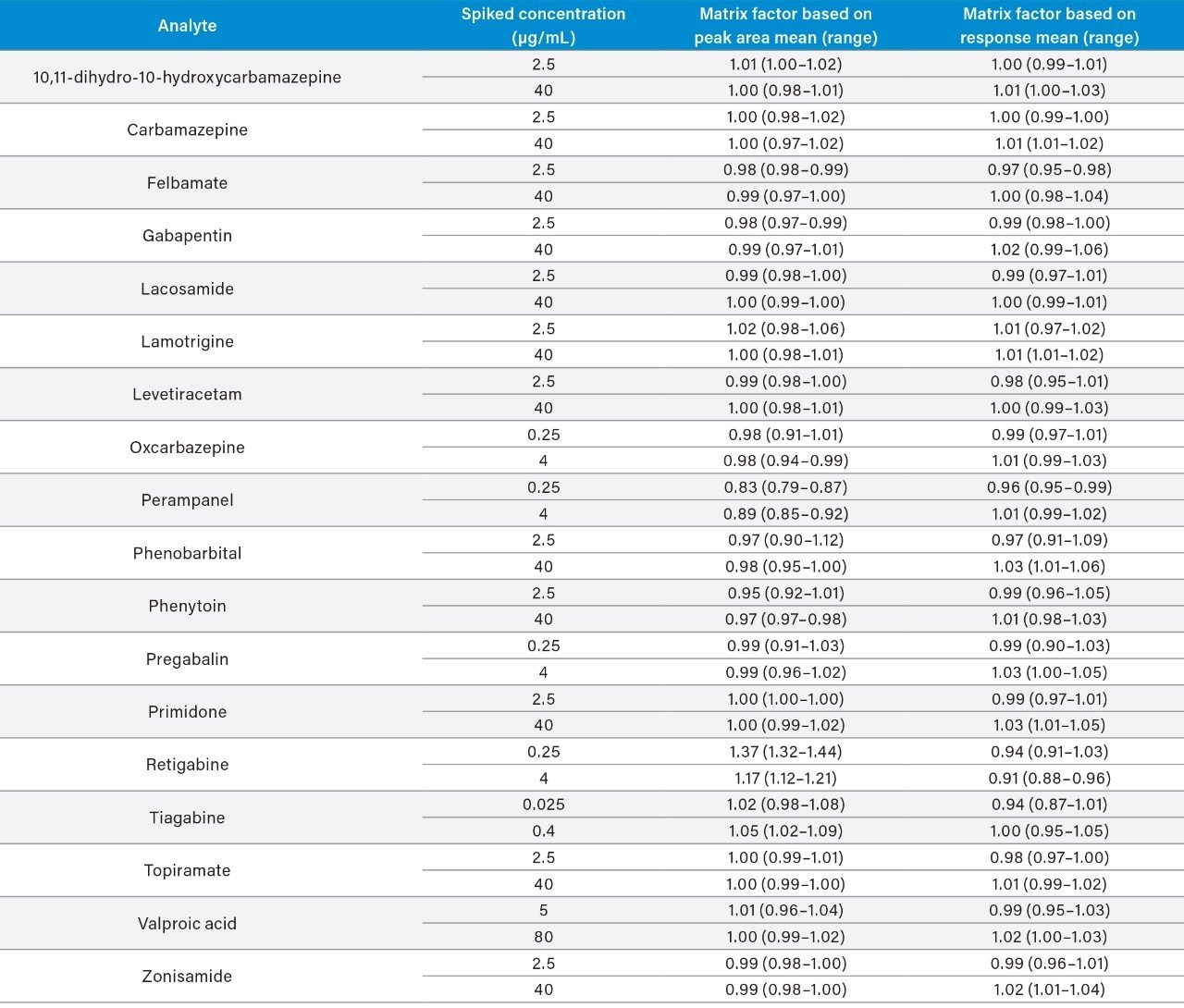
Matrix effects were evaluated at low (QC1) and high (QC3) concentrations in plasma (n=6) taken as a percentage of extracted solvent samples spiked to equivalent concentrations. Calculations using analyte:internal standard response ratio indicated compensation for signal enhancement by the internal standard (Table 5).
Potential interference from endogenous compounds (albumin, bilirubin, cholesterol, triglycerides, and uric acid) spiked at high concentrations was assessed by determining the recovery (n=3) from low and high pooled plasma samples (QC1 and QC3 concentrations). Recoveries ranged from 85.1–112.8%. A substance was deemed to interfere if a recovery range of 85–115% was exceeded. Additionally, full chromatographic resolution of the metabolite carbamazepine epoxide from isobaric oxcarbazepine was established.
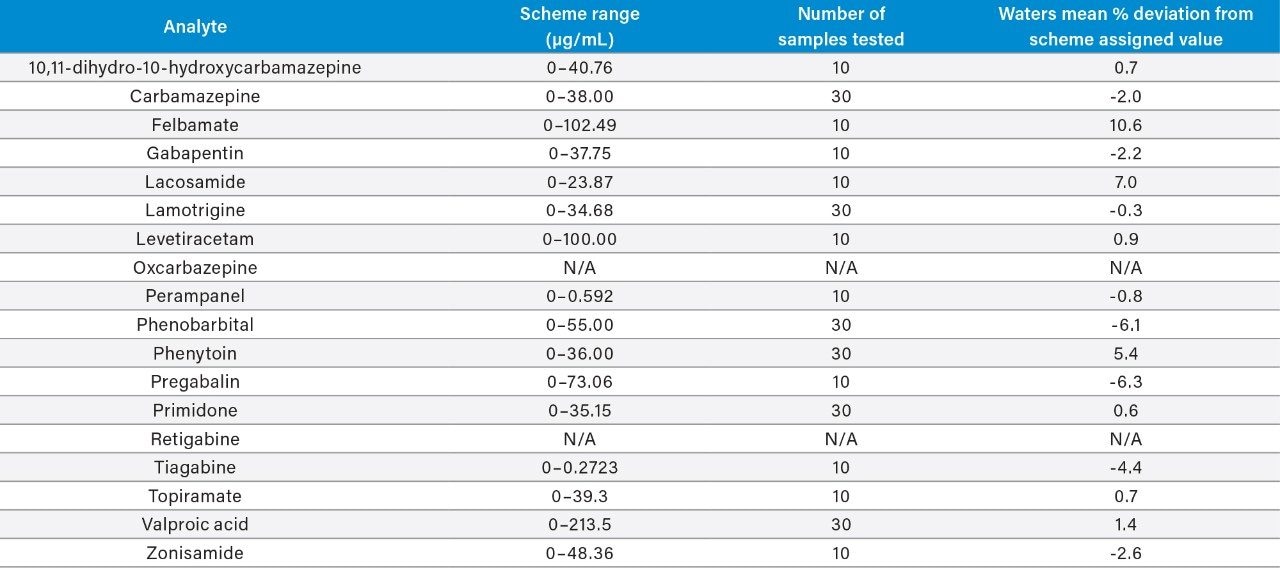
LGC (Greater London, UK) provided serum external quality assurance samples for accuracy testing, except for oxcarbazepine and retigabine, which were not included in the schemes. All samples passed the criteria of the scheme, with mean deviations ≤10.6% from assigned concentrations. Results are presented in Table 6.
The developed method for clinical research demonstrates the capabilities of the sample preparation and UPLC-MS/MS system to quantify 18 antiepileptic drugs and metabolites in plasma. The method demonstrated excellent performance characteristics, including precision and agreement with an external quality assurance scheme, with neither system carryover nor significant matrix effects.
720006788, March 2020