
This is an Application Brief and does not contain a detailed Experimental section.
Here we describe a simple modified QuEChERS extraction protocol and simple clean-up strategies suitable for multiresidue mycotoxins analysis by UPLC-MS/MS.
A simple modified QuEChERS extraction protocol and simple clean-up strategies suitable for multiresidue mycotoxins analysis by UPLC-MS/MS.
Mycotoxins are toxic compounds produced by molds or other fungi that can grow on foodstuffs intended for domestic animal or human consumption. Ingestion of food containing only parts-per-billion (µg/kg) concentration of some mycotoxins may cause severe illness. Therefore, sensitive and reliable analytical methods are required to determine mycotoxins in foods and feeds. Cereal grains, such as wheat, rice, and maize are important examples of these types of foods. Many of the natural constituents of these grains are potential interferences for LC-MS/MS analysis. Although proteins and starches are removed during the QuEChERS extraction by partition, precipitation, and centrifugation, significant amounts of fat and lecithins (phospholipids) are co-extracted along with the target mycotoxins.
The presence of these co-extracted substances can lead to interference in the LC-MS analysis, contamination of the analytical column and other components of the UPLC system, and contamination of the mass-spectrometer itself. Fats have traditionally been removed from QuEChERS extracts using cumbersome hexane defatting steps or by the use of reversed-phase sorbents such as C18– silica. Although these techniques may be effective for fat removal, neither of these procedures removes phosholipids.
Pass-through clean-up with Oasis® PRiME HLB cartridge for fat and phospholipid removal and dSPE (dispersive SPE) for removal of residual sugars and other polars. The recoveries of the mycotoxins are not compromised using these cleanup protocols.
QuEChERS Extraction. Cereal grain flours were purchased at a local grocery store. A 2 g sample was weighed into a 50 mL centrifuge tube. 10 mL water and 10 mL 10:90 formic acid/acetonitrile were added and the sample was placed on automated shaker for 1 hour. Then, QuEChERS salts (contents of DisQuE pouch for CEN, pn 186006813) were added and the tube was shaken vigorously by hand for 1 minute. After centrifugation (3200 rcf for 5 minutes), a portion of the supernatant was taken for cleanup.
Clean-up. An Oasis PRiME HLB Cartridge (3 cc, 150 mg, pn 186008717) was mounted on a pre-cleaned vacuum manifold set to minimal vacuum (approx 2 psi). No cartridge conditioning is required or was performed. A 0.4 mL aliquot of the supernatant was passed-through the Oasis PRiME Cartridge and discarded. Then a 1 mL portion of the supernatant was passed through the cartridge and collected. The collected extract was then transferred to a 2 mL dSPE tube (pn 186008081) containing a mixture of sorbents . After centrifugation (1 minute at 13500 rcf), a 500 µL aliquot was taken, evaporated under a gentle nitrogen stream, and reconstituted in 250 µL of 15:85 acetonitrile/water.
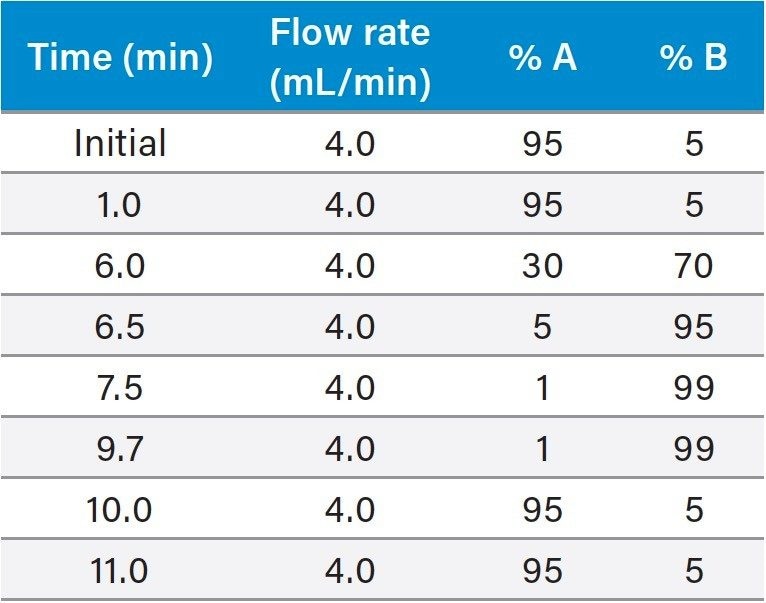
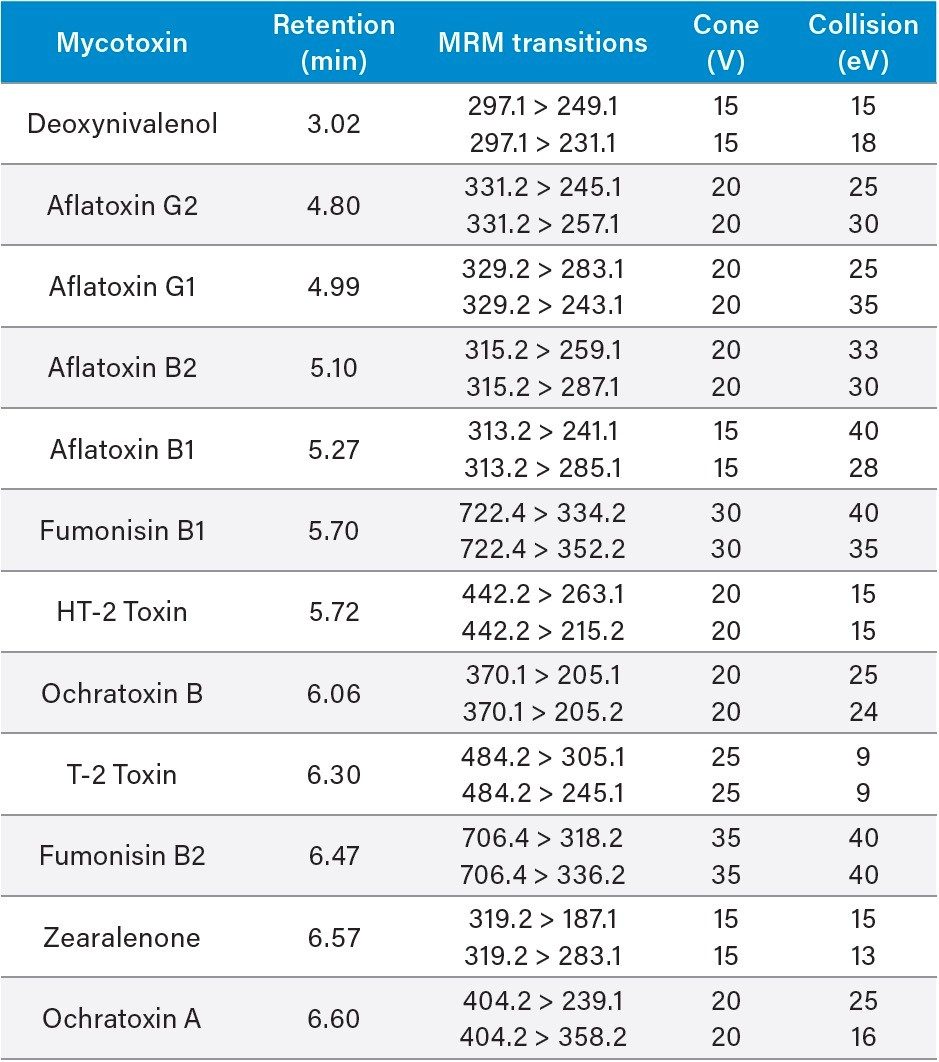
UPLC-MS/MS conditions are presented below. Table 1 presents the target compound list, MRM transitions, and mass-spectral conditions used for this study. Six point matrix-matched calibration curves run bracketing the target levels showed good linearity (R2>0.99) for all compounds.
|
LC system: |
ACQUITY UPLC I-Class (FTN) |
|
Column: |
CORTECS UPLC T3, 1.6 µm, 100 x 2.1 mm |
|
Mobile phase: |
A: 0.5% formic acid, 5 mM ammonium formate in water B: 0.5% formic acid, 5 mM ammonium formate in 50:50 methanol/acetonitrile |
|
Injection vol.: |
10 μL |
|
Column temp.: |
30 °C |
|
Needle wash and Sample manager purge: |
1% formic acid, 10 mm citric acid in 1:1:1:1 water/methanol/isopropanol/acetonitrile |
|
Seal wash: |
10:90 methanol:water |
|
Mass spectrometer: |
Xevo TQ-S micro |
|
Mode: |
Positive ion electrospray |
|
Source temp.: |
150 °C |
|
Desolvation temp.: |
500 °C |
|
Desolvation gas flow: |
1000 L/Hr |
|
Cone gas flow: |
30 L/Hr |
|
Data management: |
MassLynx v4.1 |
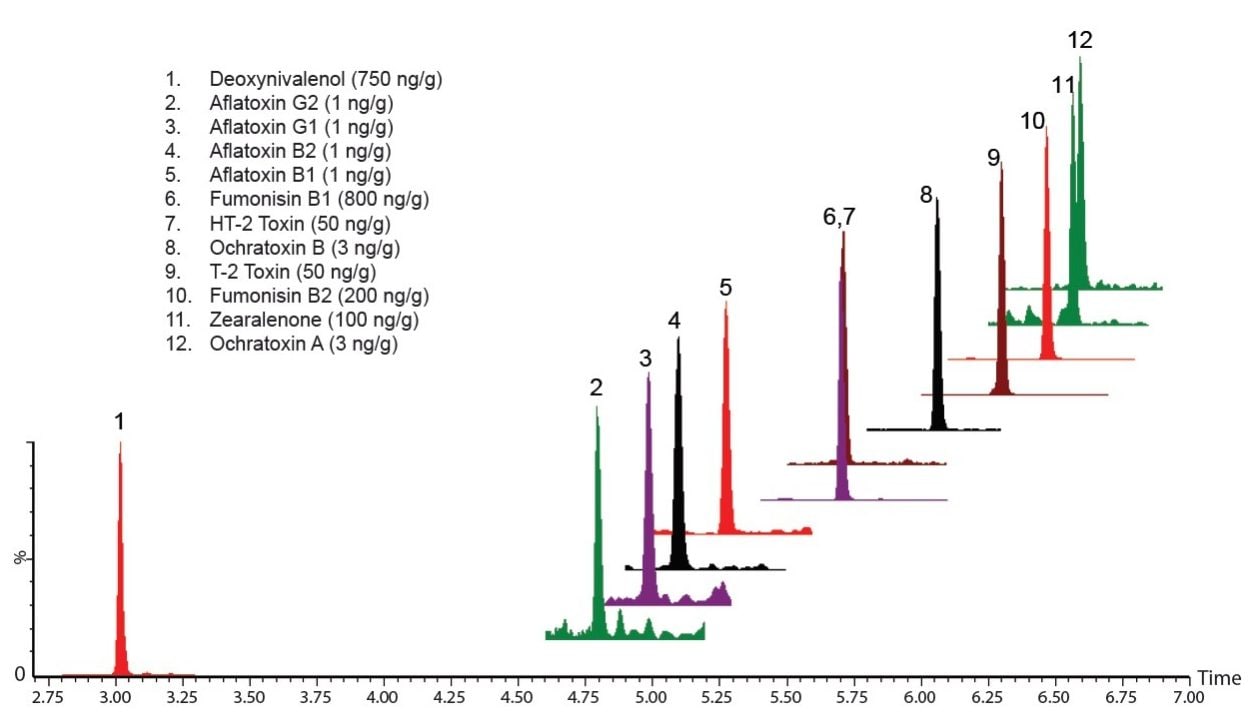
Recoveries were measured for 12 mycotoxins at a low and high level. The high level was consistent with EU maximum permitted levels for aflatoxins, fumonisins, ochratoxin A, and zearalenone, and recommended levels for T2 and HT2 toxins (see Figure 1). The low level was 1/4 X the high level (0.25 ng/g for aflatoxins). In wheat flour, total method recoveries for both the low and high level spiked samples were better than 80% for all target compounds except for zearalenone (73%). Similar performance was observed for whole rice and maize flours.
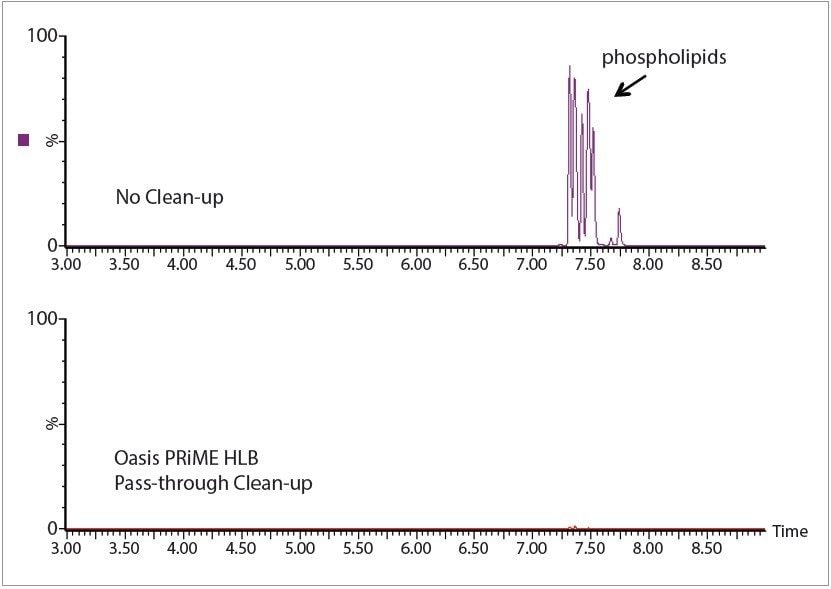
Very little of any recovery loss for any of the toxins was caused by the pass-through or dSPE clean-up steps. Figure 1 shows ion chromatograms obtained from a sample spiked at 0.25 ng/g (ppb) in wheat flour. Figure 2 shows chromatograms that illustrate the effectiveness of the Oasis PRiME Cartridge for phosholipid removal; greater than 95% of phospholipids and greater than 80% of total fats were removed using the Oasis PRiME Cartridge.
720005893, March 2018