This is an Application Brief and does not contain a detailed Experimental section.
This application brief demonstrates to assess the ability of the ACQUITY UPLC H-Class Bio, ACQUITY Arc Bio, and Alliance liquid chromatography (LC) systems to accurately deliver a quaternary ion exchange chromatography (IEX) pH gradient calculated using Auto•Blend Plus for reproducible lysine charge variant determination of a monoclonal antibody (mAb).
The Alliance, ACQUITY Arc Bio, and ACQUITY UPLC H-Class Bio systems can effectively deliver quaternary IEX pH gradients to reproducibly separate lysine charge variants of the mAb infliximab.
Ion exchange chromatography (IEX) is used in the biopharmaceutical industry as a method of characterizing protein species by isolating charge variants. This separation method uses salt or pH gradients (or both) to separate proteins based on differences in charge. These differences in charge can be a result of protein degradation, conformation, sequence variants, and post-translational modifications including glycosylation.
One method for IEX separations includes using the Waters Auto•Blend Plus function of the Empower Chromatography Data Software to generate a salt or pH gradient from a concentrated stock of buffers. The functionality of Auto•Blend Plus has previously been demonstrated in the Application Note Charge Variant Analysis of Therapeutic Monoclonal Antibodies Using a pH Gradient Generated by Auto•Blend Plus (720004906EN). The method described in this Application Note uses an ACQUITY UltraPerformance Liquid Chromatography (UPLC) H-Class Bio System to deliver both salt and pH quaternary gradients for the separation of lysine charge variants of the monoclonal antibody (mAb) infliximab.
Waters recently introduced a biocompatible version of the ACQUITY Arc System. The ACQUITY Arc was originally launched in 2016, and was designed as a stepping stone in performance between the ACQUITY UPLC H-Class Bio System and the high performance liquid chromatography (HPLC) Alliance System. The ACQUITY Arc Bio System uses a titanium and MP35N alloy flow path, making it ideal for highly corrosive separations under high salt or extreme pH conditions such as those found in IEX chromatography.
In this study, the ability of the ACQUITY Arc Bio to deliver a pH gradient for the separation of lysine charge variants of infliximab is compared with the ACQUITY UPLC H-Class Bio and Alliance Systems. Both ACQUITY systems use a gradient generated with Waters’ Auto•Blend Plus Technology, while the Alliance System replicates this gradient without the use of Auto•Blend Plus.
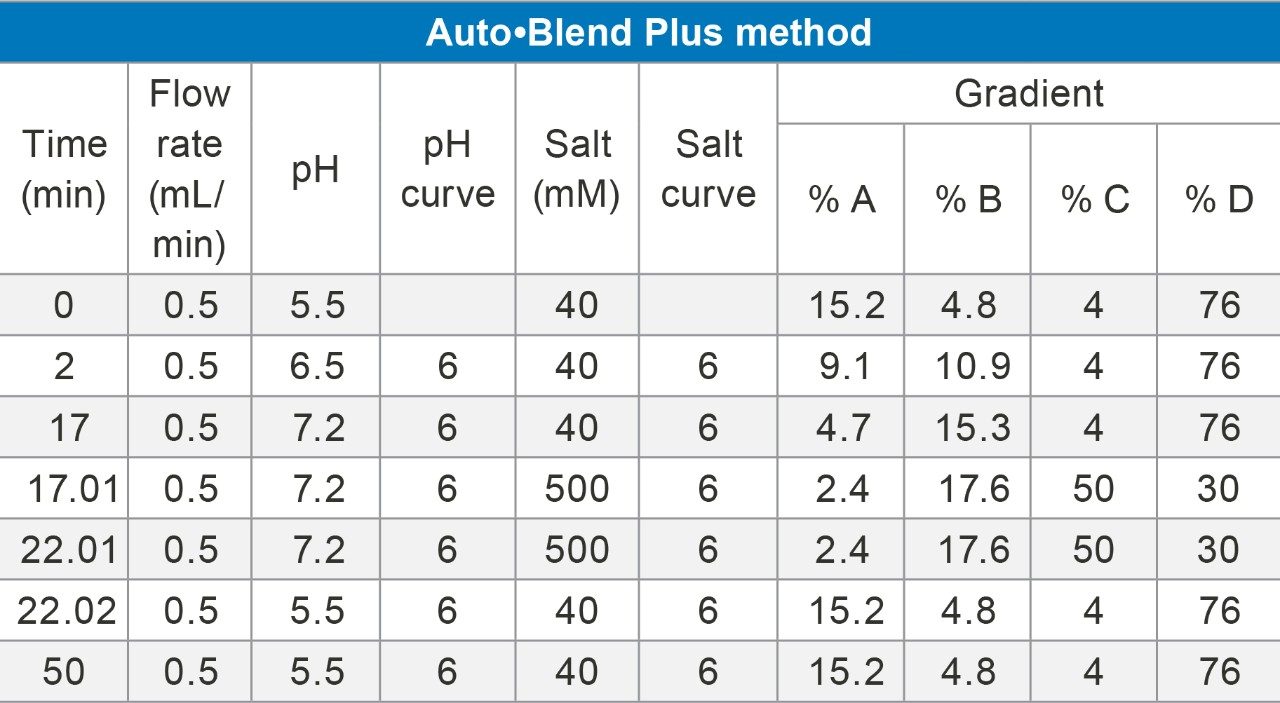
The Auto•Blend Technology used to generate a pH gradient requires four reservoirs of stock solution: concentrated acid, concentrated base, concentrated salt, and pure water for dilution. The mobile phases used, chromatography conditions, the Auto•Blend Plus method, and the resulting gradient method are given in Table 1. These conditions follow closely with the methods presented in the aforementioned Application Note with two minor changes: a two minute hold has been introduced before the gradient initiates to allow the column to better re-equilibrate following the sample injection, and the column re-equilibration following the pH gradient has been extended to ensure starting mobile phase conditions were reached for the next injection. Both of these changes resulted in improved retention time reproducibility.
Five injections were made on each chromatographic system. The retention time reproducibility of these five injections is visually depicted in Figure 1. All three systems displayed excellent chromatographic profile reproducibility over the five mAb injections. The three main lysine variant peaks were used to measure retention time reproducibility on all three systems (Table 2). The ACQUITY Arc Bio System demonstrated the lowest retention time standard deviations while the ACQUITY UPLC H-Class Bio System demonstrated the highest retention time standard deviations for all peaks. The relatively lower retention time reproducibility of the ACQUITY UPLC H-Class Bio is presumed to be due to a combination of the low column back-pressure of the separation and the active check-valve design of that LC system.
In order for an analytical method to be transferred between chromatographic systems or labs, the method needs to be reproducible across the systems in question. In the case of IEX lysine charge variant analysis of infliximab, the measure of importance is the level of each charge variant present in solution. Relative area counts of the three lysine charge variant peaks are given in Table II. The profiles across all three systems are visually comparable (Figure 1), as are the relative retention times and abundances of the charge variant peaks. This speaks to the robustness of the chromatographic systems, columns, and methods used.
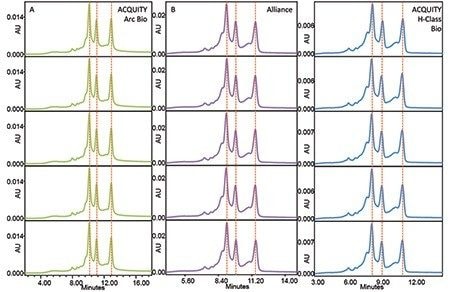

Ion exchange chromatography is used in the biopharmaceutical industry as a method of protein variant analysis. Auto•Blend Plus was used to generate a pH gradient on the ACQUITY systems (ACQUITY UPLC H-Class Bio and ACQUITY Arc Bio) and replicated on the Alliance System. All three systems exhibited good retention time reproducibility of the three major lysine variant peaks in the infliximab chromatographic profile. The ACQUITY Arc Bio achieved the lowest retention time standard deviation. Additionally, relative lysine variant quantification on all three systems was very comparable.
720006414, October 2018