The ACQUITY UPLC Columns Calculator is a useful tool when scaling methods by providing the user with scaled flow rates, injection volumes, and gradient tables when applicable. In this example for the analysis of fluconazole and fluconazole related compounds A, B, and C, the run time was decreased by more than 65%, and solvent usage was decreased by more than 85%. Additionally, the ACQUITY UPLC I-Class system provides superior chromatographic performance (due to the lower volume of dispersion over that of UHPLC systems) with increased resolution, increased theoretical plates and more symmetrical peak shape.
Increased throughput by scaling methods from HPLC to UPLC, improved chromatographic performance with a lower dispersion instrument.
Many current USP methods are designed for use with larger particle size (3 μm or greater) HPLC columns and instrumentation, which results in long run times and large volumes of hazardous solvents being consumed. It is possible to scale these methods to sub-2-μm particle columns, which provides the same or improved performance with shorter run times resulting in increased throughput and lower solvent consumption. In order to scale methods appropriately, a number of USP guidelines must be followed.1 For example, the L/dp ratio, where L refers to the length of the column and dp refers to the diameter of the particles packed within the column, must remain within -25% to +50% of the original HPLC method. It is critical to be aware of these regulatory requirements when scaling from HPLC to UPLC methodologies.
In addition to method parameters, individual instrument characteristics will also affect the resulting chromatography. Often, system suitability requirements will include resolution between a critical pair of compounds. Extra column dispersion is an instrument characteristic which will greatly impact the resolution between peaks. This is due to an increase in band broadening as the dispersion of a system increases.2 In this application note the USP monograph for fluconazole and related impurities3 will be scaled from the original HPLC method to a UPLC method and will be run on multiple instruments to show the differences in performance due to individual instrument characteristics.
Fluconazole and fluconazole related compounds A, B, and C were purchased from United States Pharmacopeia. Samples were initially dissolved in acetonitrile, then diluted with 80:20 (v:v) water: acetonitrile per the USP monograph for fluconazole. Samples were vortexed and sonicated to ensure complete dissolution. The final concentration of the sample was of 10 μg/mL for all reference compounds.
|
LC conditions |
|
|
LC systems: |
Competitor UHPLC 1 System Competitor UHPLC 2 System ACQUITY UPLC I-Class System with CH-A |
|
Detection: |
Competitor DAD 1 for UHPLC System 1, Competitor DAD 2 for UHPLC System 2, and ACQUITY UPLC PDA Detector |
|
Sample: |
Fluconazole and fluconazole related compounds A, B, and C (USP catalog numbers 1271700, 1271711, 1271722, and 1271733 respectively) |
|
Column: |
ACQUITY UPLC HSS T3, 1.8 μm, 2.1 x 75 mm |
|
Column temperature: |
40 °C |
|
Mobile phase A: |
Water |
|
Mobile phase B: |
Acetonitrile |
|
Mobile phase composition: |
80:20 Mobile phase A: Mobile phase B |
|
Flow rate: |
0.200 mL/min |
|
Injection volume: |
2.1 μL |
|
Wavelength: |
260 nm |
|
Collection rate: |
10 Hz |
|
Needle wash: |
90/10 Methanol/Water |
|
Seal wash: |
80/20 Water/Methanol |
|
Chromatography software: |
Empower 3 FR2, Chromeleon 7.2 |
The USP HPLC method for the analysis of fluconazole and related compounds was scaled to a UPLC method using the ACQUITY UPLC Columns Calculator (Figure 1). Scaling from a 4.6 x 150 mm, 3.5 μm column to a 2.1 x 75 mm 1.8 μm column results in a L/dp ratio decrease of 2.7%, which is well within the USP criteria (-25% to +50% of the original method L/dp). The flow rate, scaled accounting for particle size, decreased from 0.500mL/min in the original method to 0.200 mL/min in the updated method, and the injection volume decreased from the original value of 20 μL to 2.1 μL. The appropriately scaled method was then run on the ACQUITY UPLC I-Class System, as well as two competitive binary UHPLC systems (Figure 2). Scaling the method to a UPLC column resulted in much faster peak elution, requiring less than 4 minutes, as compared to the initial HPLC method, in which the last peak required 10 min for elution.3 The chromatogram acquired on the UPLC instrument shows peaks which are narrower, and thus have a higher peak height than the same sample run on the UHPLC instruments. The most notable example is the earliest eluting peak, fluconazole related compound A, where the peak height is more than 1.5x greater for the UPLC chromatogram as compared to UHPLC Competitive System 1, and 1.7x greater than the UHPLC Competitive System 2.
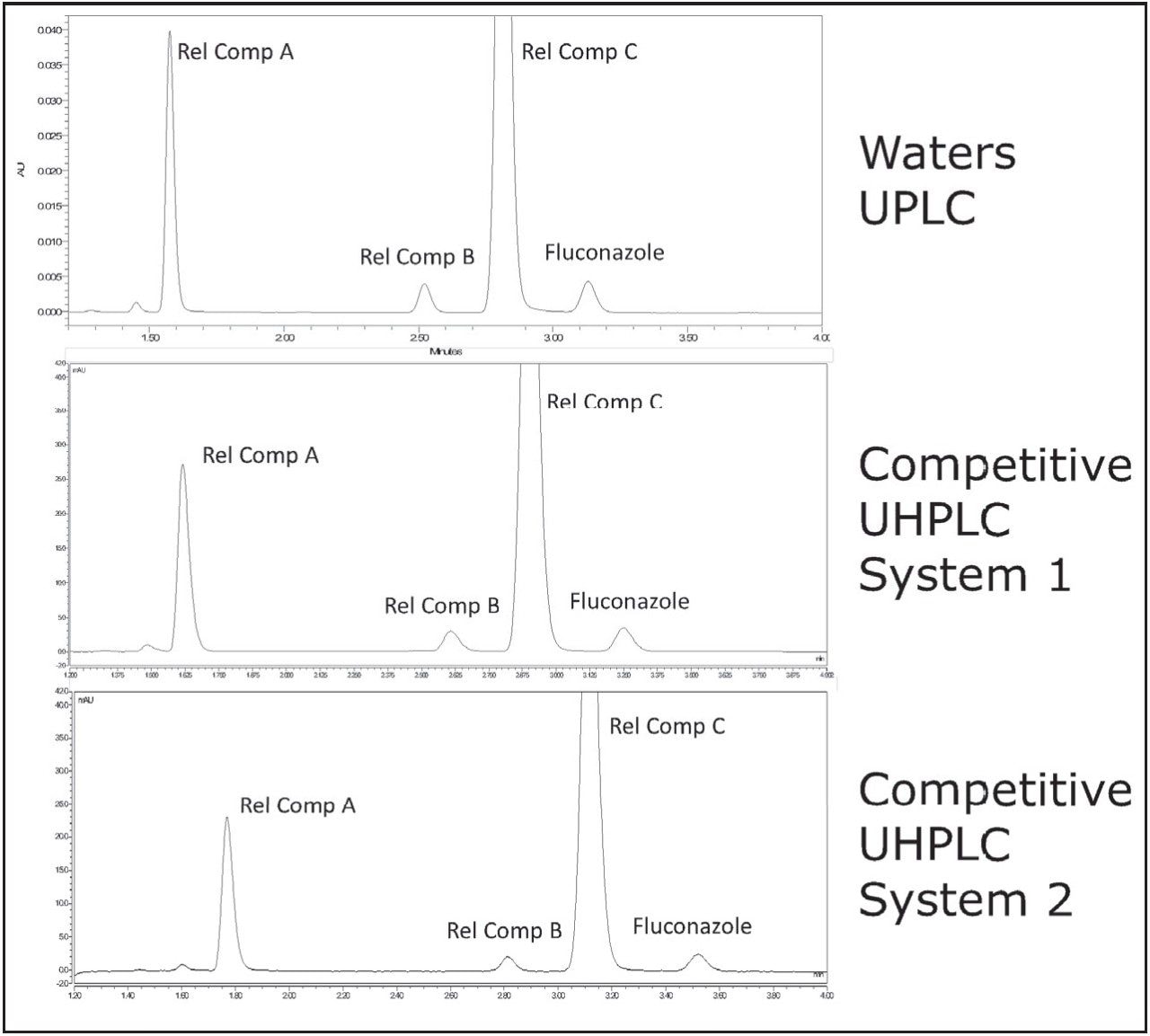
System suitability requirements for fluconazole and fluconazole related compounds A–C state that resolution between related compound B and related compound C must be NLT (not less than) 1.5. Additionally, relative standard deviation must be NMT (not more than) 5.0% for each peak. The results obtained on the ACQUITY UPLC I-Class and the two UHPLC systems are listed in Table 1. All three of the systems tested were able to meet acceptance criteria for system suitability.
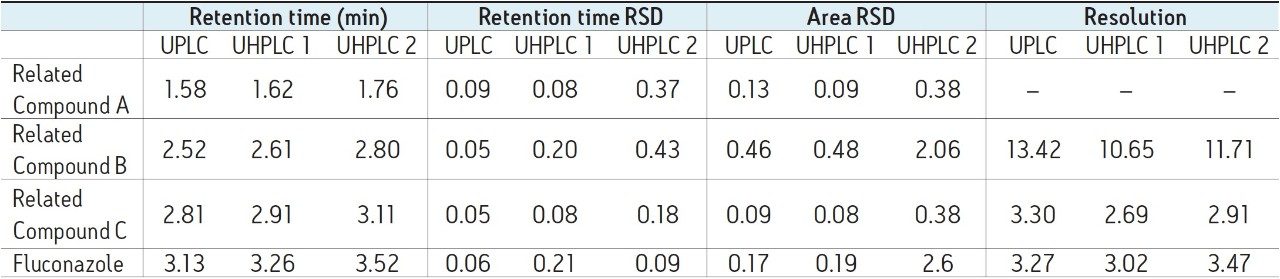
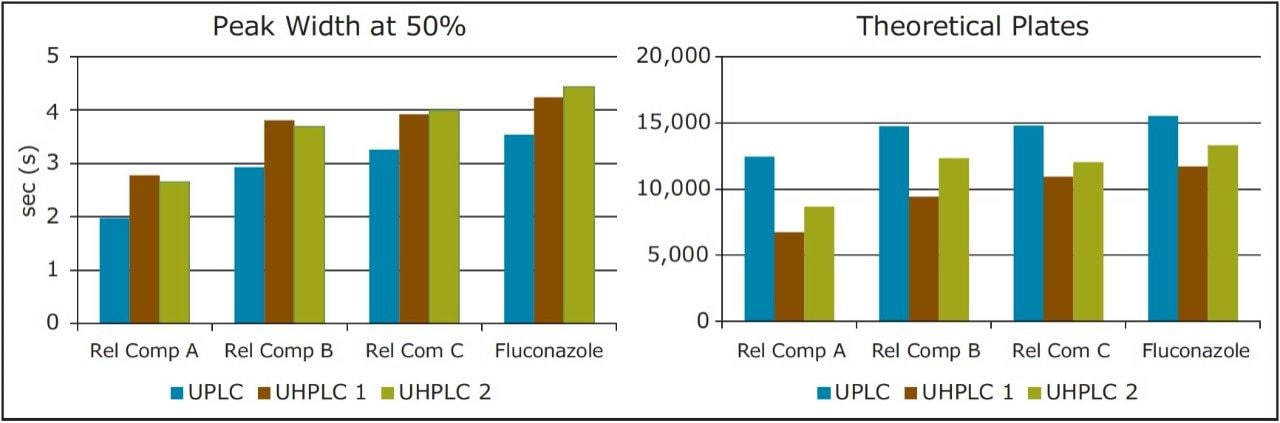
There are, however, differences in chromatographic performance between the three systems, largely due to dispersion. Extra column dispersion measurements indicated the ACQUITY UPLC I-Class system has a value of approximately 1/2 that of the two UHPLC systems. Due to this, the UPLC ACQUITY UPLC I-Class system, with the lower dispersion, provides sharper, narrower peaks. This also correlates to an increase in the efficiency and thus a greater number of theoretical plates (Table 2). This effect is most noticeable for compounds with a shorter retention time, or a relatively small k'. In this example, fluconazole related compound A is the earliest eluting compound, and shows the largest discrepancy in measured plates between the two systems. It is also apparent from the chromatogram that this first peak suffers the most from peak broadening and thus will exhibit a decrease in peak height. This drop in chromatographic performance is mainly an artifact of the increased dispersion volume in the UHPLC systems as compared to the ACQUITY UPLC I-Class UPLC system.
The ability to scale traditional HPLC methods to modern UPLC methods provides equivalent or enhanced performance while decreasing run time and solvent consumption. The ACQUITY UPLC Columns Calculator is a useful tool when scaling methods by providing the user with scaled flow rates, injection volumes, and gradient tables when applicable. In this example for the analysis of fluconazole and fluconazole related compounds A, B, and C, the run time was decreased by more than 65%, and solvent usage was decreased by more than 85%. Additionally, the ACQUITY UPLC I-Class system provides superior chromatographic performance (due to the lower volume of dispersion over that of UHPLC systems) with increased resolution, increased theoretical plates and more symmetrical peak shape.
720005589, January 2016