
This is an Application Brief and does not contain a detailed Experimental section.
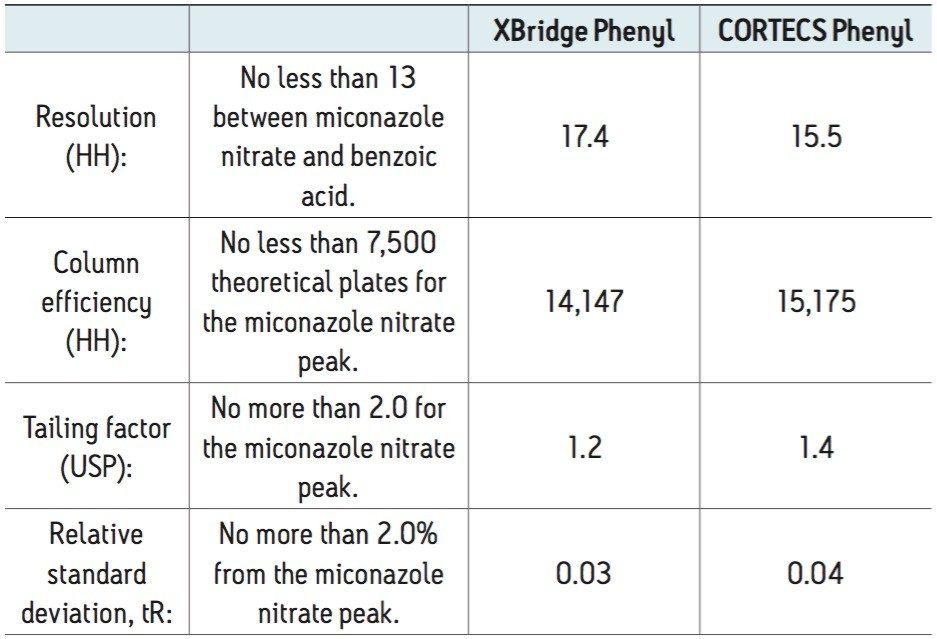
This application brief demonstrates that smaller configuration, high efficiency solid-core CORTECS Phenyl 2.7 μm Columns can be used to modernize methods that use larger, 5 μm HPLC columns. Comparable chromatographic selectivity and enhanced resolution was achieved with these columns to meet the criteria for an official USP monograph.
Older USP methods typically use longer HPLC column lengths packed with larger diameter fully-porous particles. The modernization of a USP method can be achieved by reducing both column dimension and particle size. Solid-core particle packed columns offer higher efficiency per unit length, a distinct advantage over fully-porous particles of the same size.
USP methods that were developed for traditional HPLC systems have typically used columns packed with relatively large diameter, fully-porous particles. Revalidation of these methods to include the use of high performance ACQUITY UPLC Systems and modern, more efficient HPLC columns can be costly and time consuming. However, isocratic USP methods can be modernized in accordance to the recent changes in USP General Chapter <621>, which allow for scaling of constant L/dp (column length per particle diameter) or -25% to +50% column efficiency. In this Technology Brief, an updated analysis for miconazole nitrate cream, using a CORTECS Phenyl 2.7 μm Column, is described. The modernization was achieved by reducing column dimension and particle size within the USP <621> allowed limits.
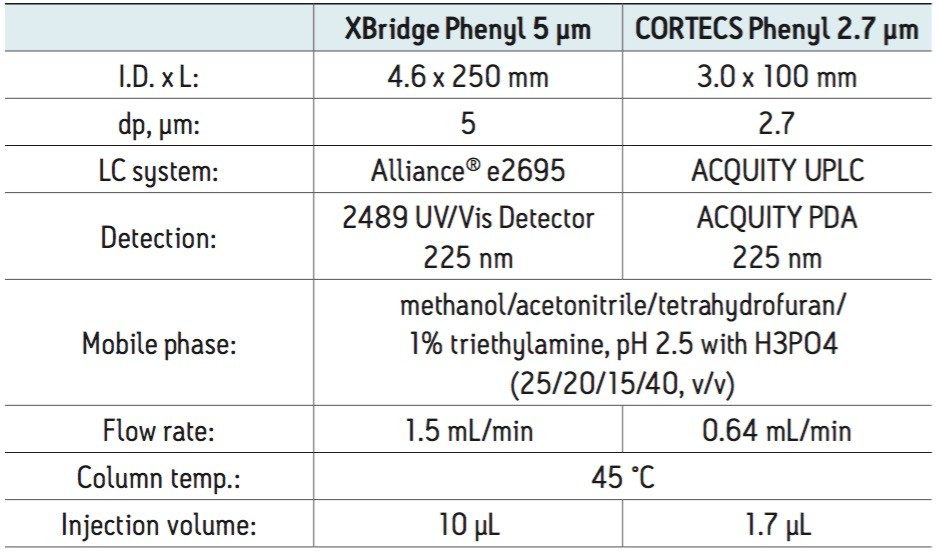
A CORTECS Phenyl Column, 2.7 μm, 3.0 x 100 mm was compared to an XBridge Phenyl Column, 5 μm, 4.6 x 250 mm , the recommended L11 column configuration and particle size for the separation of miconazole nitrate and benzoic acid, an anti-microbial preservative. The separation was achieved using the quaternary mobile phase system of methanol/acetonitrile/tetrahydrofuran/1% triethylamine, pH 2.5 (25/20/15/40, v/v) as given in the USP monograph.
A standard solution of 0.28 mg/mL of miconazole nitrate and 0.02 mg/mL of benzoic acid were prepared in mobile phase. A sample solution with a concentration of 0.28 mg/mL of miconazole nitrate and 0.02 mg/mL of benzoic acid was prepared by dispersing one applicator of an OTC (over-the- counter) cream preparation containing 100 mg of miconazole per dose in 357 mLs of mobile phase. The solution was heated for one hour at 40–45 °C in an ultrasonic bath, and then allowed to cool to room temperature. Twenty milliliters was filtered through a 0.45 μm Teflon filter, and a 2 mL aliquot was transferred to a TruView LCMS Clear Glass Vial for analysis.
The suitability measurements were determined using the standard solutions and an XBridge Phenyl Column, 5 μm, 4.6 x 250 mm, on an HPLC system, and were determined for the CORTECS Phenyl Column, 2.7 μm, 3.0 x 100 mm on a UPLC system. The chromatographic conditions for both columns are given in Table 1.
The high efficiency of CORTECS Phenyl Columns allows for the use of a smaller configuration column, reducing the analysis time threefold without the need for revalidation. The change in configuration did not impact the system suitability results (Table 2).
According to the USP method, the nominal amount of miconazole nitrate in the cream formulation is 0.28 mg/mL. Five injections of the sample solution were made on each column using the volumes given in Table 1. The percentage of the labeled amount of miconazole nitrate in the cream was calculated using the following formula:
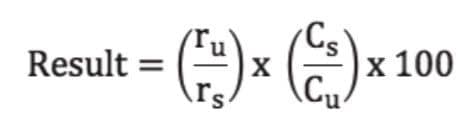
ru = peak response (area) from sample solution
rs = peak response (area) from the standard solution
Cs = concentration of miconazole nitrate in standard solution
Cu = nominal concentration of miconazole nitrate in the sample solution
The acceptance criterion for the labeled amount is 90.0%-110.0% of the listed amount. The result for the XBridge Phenyl Column, 5 μm, 4.6 x 250 mm was 96.3% and for the CORTECS Phenyl Column, 2.7 μm, 3.0x 100 mm, the result was 94.9%. The miconazole nitrate peak height on the more efficient CORTECS Phenyl 2.7 μm Column was 35% higher than on the XBridge Phenyl 5 μm Column. Figure 1 shows the separation of the prepared miconazole nitrate sample on each column.
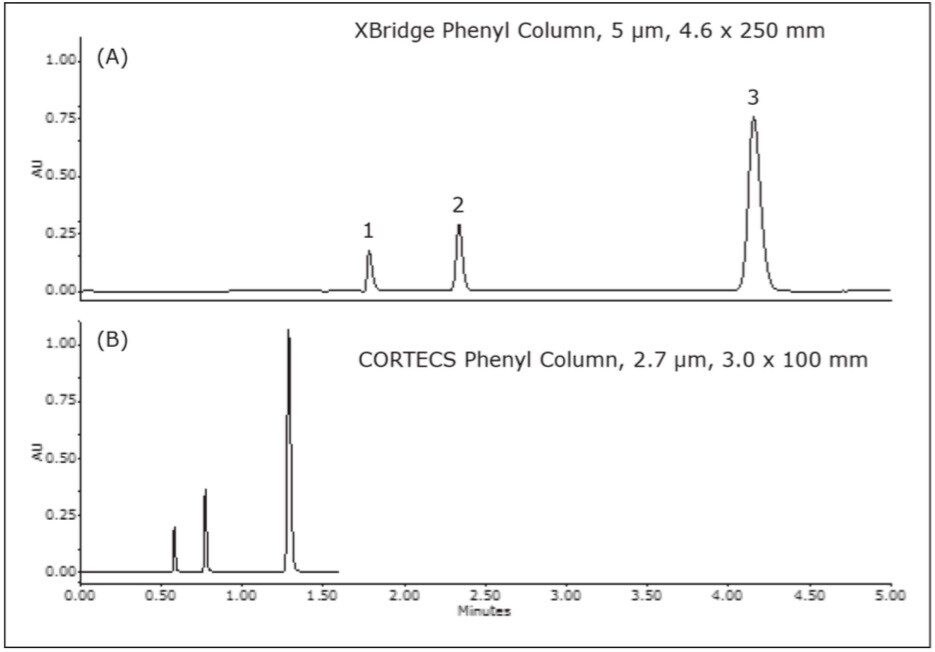
CORTECS Phenyl Columns deliver similar selectivity as other USP L11 designated columns, such as an XBridge Phenyl Column, allowing for modernization of USP methods. By utilizing the allowed limits for column length and particle diameter given in USP General Chapter <621>, the published miconazole nitrate method was performed three times faster with 35% higher sensitivity. The rapid analysis that met the USP method requirements of the miconazole nitrate cream monograph was easily achieved with a combination of the high performance ACQUITY UPLC System and high efficiency CORTECS Phenyl 2.7 μm Columns.
720005622, March 2016