In this application note, the separation of tioconazole and tioconazole related compounds A, B, and C is demonstrated using the USP organic impurities method specified in the USP monograph on several different column dimensions.
Organic impurities in generic drugs are routinely analyzed by pharmaceutical manufacturers worldwide. Performing organic impurities analyses with older instrumentation and column technology can be a time-consuming and costly task, as these methods require large amounts of solvent over extended time periods. However, organic impurities assays can become more efficient by using significantly improved instrumentation and column technology. eXtended Performance (XP) columns are 2.5-μm particle size columns designed for use on both HPLC and UPLC instrumentation. These columns are ideal for modernizing USP methods as they allow chromatographers to realize the benefit of smaller particle sizes and low dispersion systems, while operating under the USP Chapter <621> Chromatography guidelines. The <621> chapter outlines allowable changes to the method.
Tioconazole is an imidazole antifungal compound used in the treatment of yeast infections. The method that was transferred was the organic impurities analysis of tioconazole.2 Organic impurity methods are used to determine the presence and quantity of impurities in a sample. The USP method was scaled from the original column dimensions on an HPLC system to XP columns on both HPLC and UPLC instrumentation. Updating the current USP method using XP columns on an HPLC instrument can reduce run times, increasing sample throughput in a routine analysis laboratory, while using XP columns on a UPLC system can further reduce run time and solvent usage compared to HPLC, resulting in overall cost savings.
|
Mobile phase: |
44:40:28 acetonitrile/ methanol/water with 2 mL ammonium hydroxide |
|
Separation mode: |
Isocratic |
|
Detection: |
UV at 219 nm |
|
Column (L1): |
XSelect CSH C18, 4.6 x 250 mm, 5 μm, p/n 186005291; XSelect CSH C18 XP, 4.6 x 150 mm, 2.5 μm, p/n 186006729; XSelect CSH C18 XP, 4.6 x 100 mm, 2.5 μm, p/n 186006111 |
|
Column temp.: |
5 °C |
|
Needle wash: |
95:5 ACN/water |
|
Sample purge: |
95:5 water/ACN |
|
Seal wash: |
50:50 MeOH/water |
|
Flow rate: |
Scaled with method |
|
Injection volume: |
Scaled with method |
|
Mobile phase: |
44:40:28 acetonitrile/ methanol/water with 2 mL ammonium hydroxide |
|
Separation mode: |
Isocratic |
|
Detection: |
UV at 219 nm |
|
Column (L1): |
XSelect CSH C18 XP, 4.6 x 150 mm, 2.5 μm, p/n 186006729; XSelect CSH C18 XP, 4.6 x 100 mm, 2.5 μm, p/n 186006111; XSelect CSH C18 XP, 2.1 x 150 mm, 2.5 μm, p/n 186006727 |
|
Column temp.: |
25 °C |
|
Needle wash: |
95:5 ACN/water |
|
Sample purge: |
95:5 water/ACN |
|
Seal wash: |
50:50 MeOH/water |
|
Flow rate: |
Scaled with method |
|
Injection volume: |
Scaled with method |
|
Data management: |
Empower 3 Software |
The Tioconazole sample was prepared in 100% methanol to the concentrations described in Table 1. The sample was transferred to a TruView Maximum Recovery Vial for injection, p/n 186005662CV.
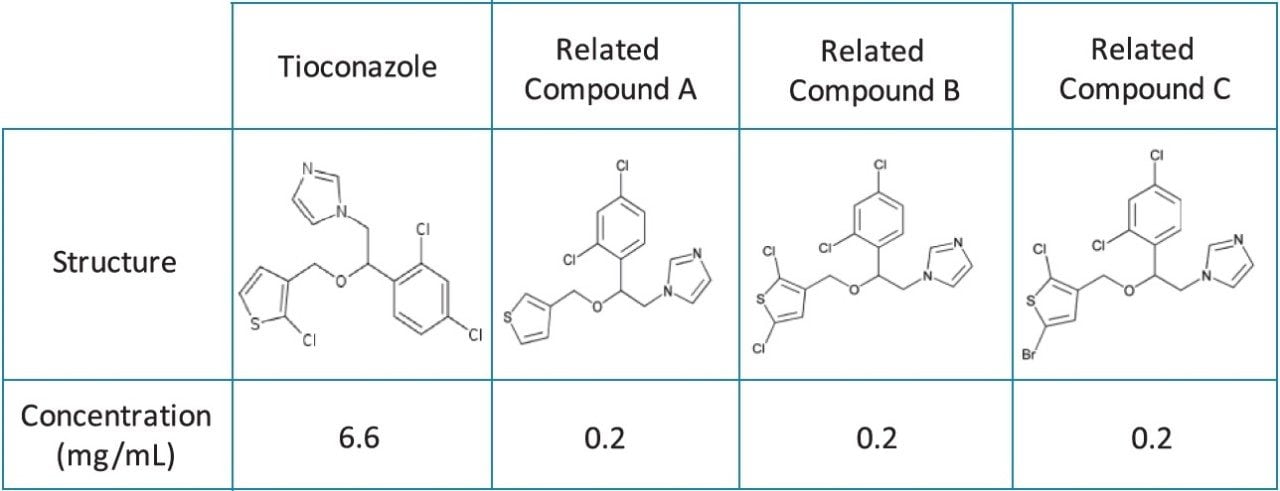
Tioconazole, produced generically, is routinely analyzed worldwide by pharmaceutical manufacturers. In this application, the separation of tioconazole and tioconazole related compounds A, B, and C is demonstrated using the USP organic impurities method specified in the USP monograph on several different column dimensions. Tioconazole related compounds A, B, and C were used as low level impurity standards, as tioconazole impurities were not readily available. Organic impurity methods listed in the USP are used to analyze complex sample formulations. The well-resolved separation of multiple components in the samples often requires the use of longer column dimensions. The use of longer columns with larger particle sizes (≥3.5 μm) results in long run times and the use of large amounts of solvent. For example, the original USP organic impurities analysis of tioconazole required a 4.6 x 250 mm, 5 μm column with the separation lasting thirty minutes, using 30 mL of solvent per sample per analysis. However, by using eXtended Performance (XP) 2.5 μm particle columns, run times may be reduced while still meeting assay requirements. With shorter run times, throughput can increase with less solvent used per analysis, leading to overall cost savings. The current USP Chapter <621> Chromatography guidelines provide allowable method changes that include ±70% change to column length, -50% change in particle size, and ±50% change in flow rate.1 These guidelines were followed throughout the method transfers demonstrated here. A USP resolution of 1.5 between related compounds B and C was used as a requirement in this application to demonstrate that this critical pair can be consistently resolved as the method is transferred between different columns and systems.
The organic impurities method for the analysis of tioconazole requires the use of an L1 designation column, and the listed column for this separation is a LiChrosorb RP-18.2 Using the Waters Reversed-Phase Column Selectivity Chart, the more modern XSelect CSH C18 stationary phase was chosen. The XSelect CSH C18 Column was chosen because of its similarity to the listed column, and it offers full scalability of dimensions and particle sizes between HPLC and UPLC instrumentation. The USP method for this separation was first run using an Alliance HPLC System with an XSelect CSH C18, 4.6 x 250 mm, 5 μm Column with a flow rate of 1.0 mL/min. The acceptance criteria for this separation were met, as shown in Table 2. The total run time for this separation was thirty minutes, which poses challenges in both time and financial management in high throughput environments where samples are continuously analyzed. Using the original USP method, an eight-hour work shift would result in only 16 samples being analyzed with 480 mL of solvent used. By using XP columns, up to 80 samples can be analyzed in the same eight-hour shift using only 240 mL of solvent, thus significantly increasing throughput and reducing operating costs.
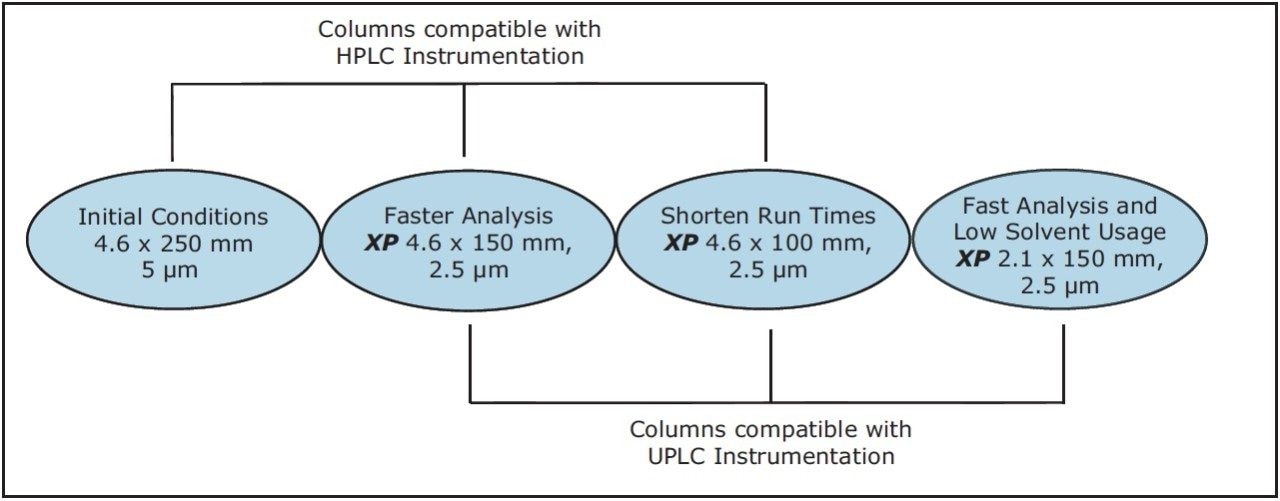
The versatility of modernizing the compendial method using XP 2.5 μm columns across different systems, while remaining within USP Chapter <621> guidelines, is shown in Figure 1. XP columns are 2.5-μm particle HPLC and UPLC columns that are packed to a high efficiency and designed to withstand the higher pressures of a UHPLC system, allowing the XP columns to run across bot HPLC and UPLC instrumentation.
The compendial method was first transferred from the original 4.6 x 250 mm, 5 μm column to an XP 4.6 x 150 mm, 2.5 μm column to demonstrate that shorter run times can be achieved by using smaller particle sizes. Using smaller particle sizes can also lead to an increase in resolving power, measured by the column length to particle size ratio (L/dp). In this case, the L/dp increases from 50,000 (initial conditions) to 60,000 when moving to an XP 4.6 x 150 mm column. According to the ACQUITY UPLC Column Calculator, the properly scaled flow rate for this column is 2.0 mL/min.3 However, that flow rate is outside the USP Chapter <621> guidelines. A flow rate of 1.0 mL/min was used to remain within USP guidelines, and to accommodate back pressure limitations of the HPLC system. The separation of tioconazole and its related compounds on the original column are compared to the XP 4.6 x 150 mm column, as shown in Figures 2A-B. The XP 4.6 x 150 mm column shows a 43% reduction in run time, along with a 5% increase in resolution, as shown in Table 2.
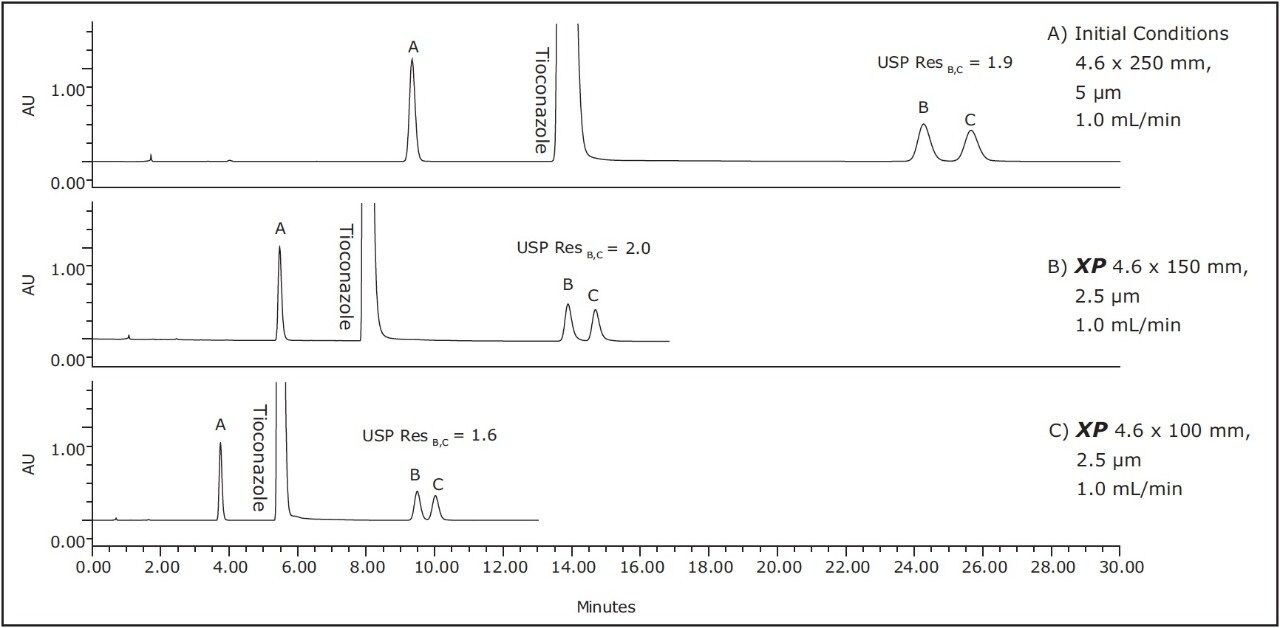
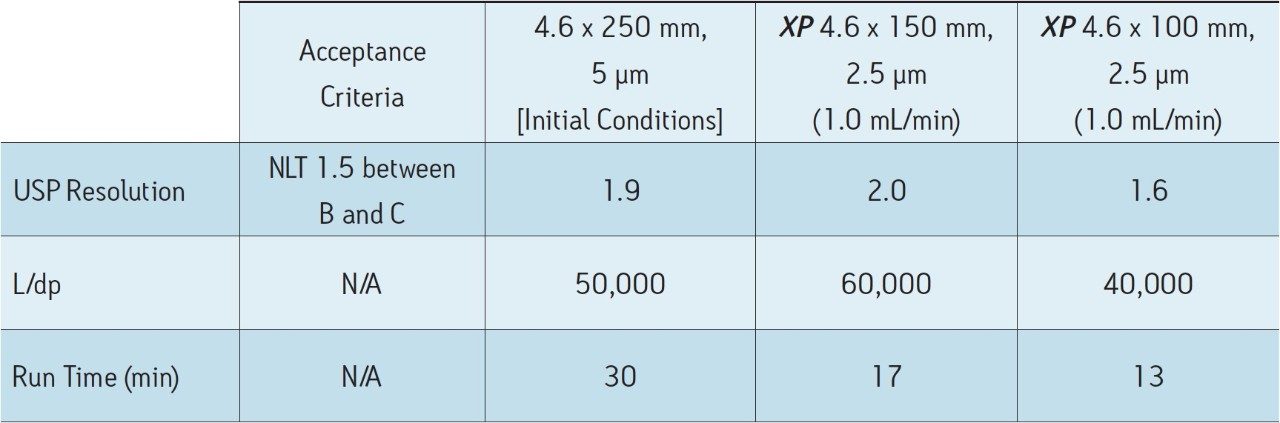
Next, the separation was performed using a shorter XP 4.6 x 100 mm, 2.5 μm column to demonstrate a faster separation while maintaining acceptable resolution. The reduced run times especially benefit organic impurity methods as these methods generally have longer run times than other methods due to added complexity of the separation. It is important to note that moving to a shorter column with lower resolving power (L/dp 40,000) may not always be an option, for instance, in cases where closely eluting excipients and impurities may require the resolving power of the original separation. Figure 2C shows that the separation using the XP 4.6 x 100 mm, 2.5 μm column results in a 57% reduction in run time compared to the initial conditions and all of the acceptance criteria are still met, as shown in Table 2. In this case, the reduction in L/dp from 50,000 (initial conditions) to 40,000 resulted in a drop in resolution between related compounds B and C of 15%; however, the resolution may still be adequate, depending on the complexity of the original separation.
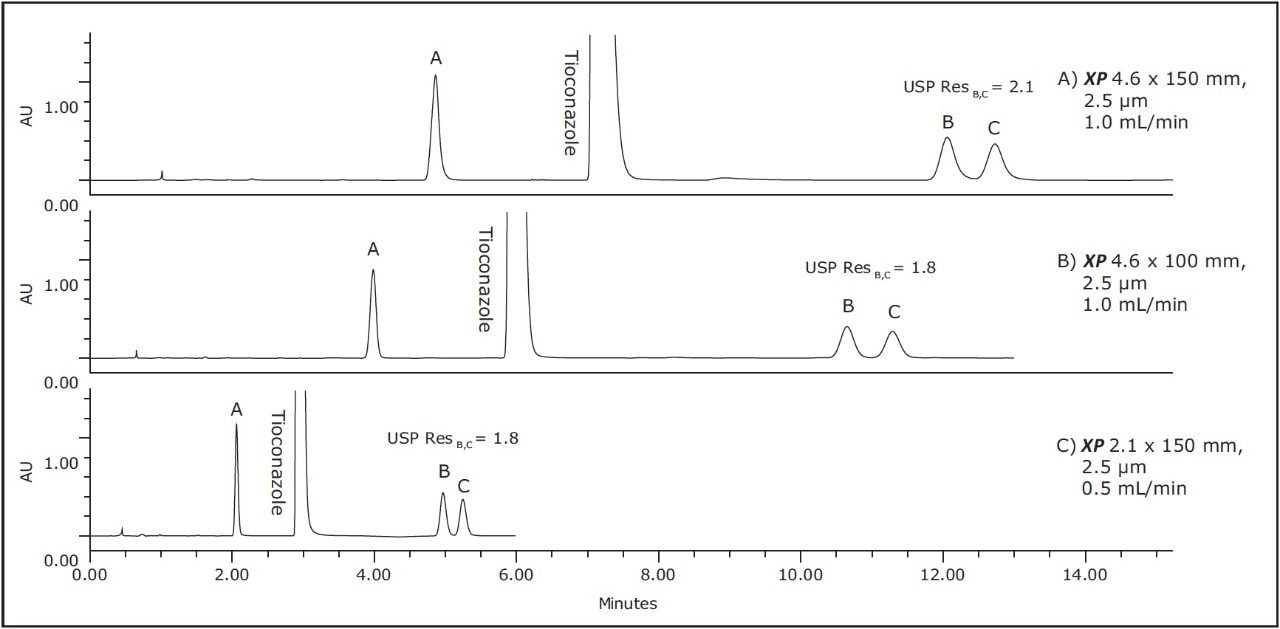
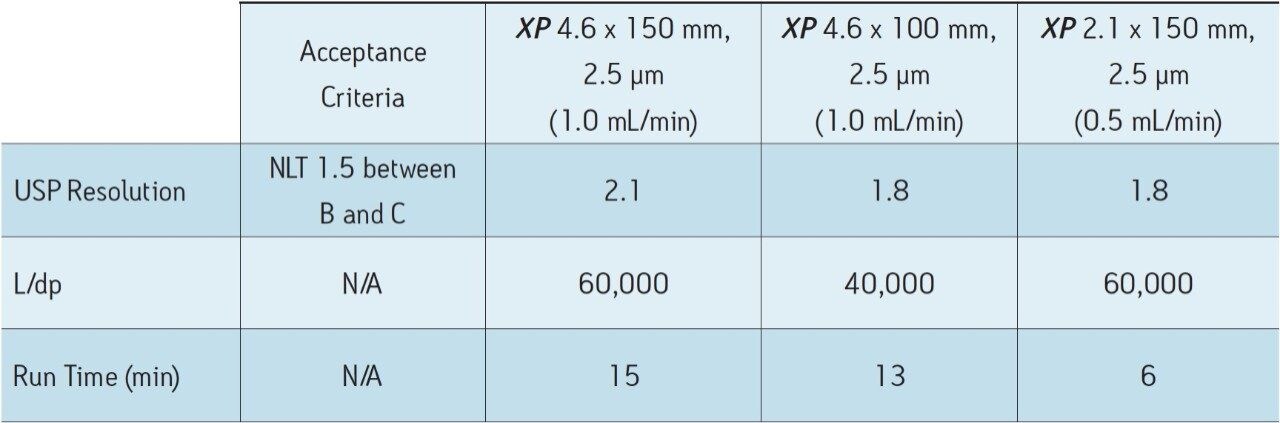
As Figure 1 outlines, the method can be transferred from an Alliance HPLC System to an ACQUITY UPLC H-Class System by using both XP columns and the ACQUITY UPLC Column Calculator. Newer instrumentation, such as the ACQUITY UPLC H-Class System, can offer faster, more efficient separations due to high back pressure capabilities, faster equilibration between injections, and significantly lower system volume and dispersion. To compare the separation capabilities of the HPLC and UPLC systems, the organic impurities method using the XP 4.6 x 150 mm, 2.5 μm particle column, shown in Figure 2B, was re-run on an ACQUITY UPLC H-Class System, shown in Figure 3A. The change in instrumentation alone, from HPLC to UPLC, resulted in a 5% increase in resolution between peaks B and C, and a 12% reduction in run time, as shown in Tables 2 and 3. This increase in resolution is due to the low system volume and low dispersion of the UPLC system, as both of these attributes can improve peak shape.
To further demonstrate the benefits of UPLC instrumentation, the separation using the XP 4.6 x 100 mm column on a UPLC system was performed, as seen in Figure 3B. This separation resulted in an increase in resolution between peaks B and C from 1.6 using an HPLC system shown in Table 2 to 1.8 using a UPLC system shown in Table 3. Using the UPLC system with an XP 4.6 x 100 mm column, the separation has approximately the same resolution as the original method on the HPLC system but performed 57% faster than the original method.
Lastly, the compendial method was transferred to an XP 2.1 x 150 mm, 2.5 μm column. This column was tested to demonstrate that by reducing column interior diameter, run times can decrease further, while maintaining comparable resolution and using considerably less solvent. According to the ACQUITY UPLC Column Calculator, the proper flow rate for the method on this column is 0.42 mL/min. However, this flow rate is outside the USP <621> guidelines; therefore, a flow rate of 0.5 mL/min was used to maintain compliance. The resulting chromatogram, displayed in Figure 3C, shows an 80% reduction in run time compared to the original conditions while suitability requirements are still easily met, as shown in Table 3. In addition, by reducing only the interior diameter of the column, the analysis is 63% faster than the XP 4.6 x 150 mm column, as shown in Figure 3A. Finally, by using the XP 2.1 x 150 mm column, solvent usage was reduced by 90% compared to the original compendial method, resulting in significant cost savings. The resolution between peaks B and C of this separation dropped from 1.9 to 1.8 as the flow rate was adjusted to remain within USP Chapter <621> guidelines, but the resolution remained within assay specifications.
When performing the often lengthy and costly analysis of organic impurities, the use of eXtended Performance [XP] 2.5 μm columns on existing HPLC systems can significantly reduce run times and solvent usage by up to 57%, compared to the original compendial USP procedure. By combining XP columns with UPLC instrumentation, run times can be reduced by up to 80%, while reducing solvent usage by 90%. The availability of XP columns, which are capable of being run on both HPLC and UPLC instrumentation, allows USP methods to be updated while following the current USP Chapter <621> guidelines. In routine analysis laboratories, modernizing USP methods using columns with smaller particle sizes can result in significant time and operating cost savings.
720004474, January 2013