This application note demonstrates how a polymer can be differentiated and characterized using Ion Mobility Spectrometry-Mass Spectrometry (IMS-MS) based on its flexibility and structure. The technique is rapid and requires very little sample preparation.
Many analytical techniques are used by the polymer industry, for example gel permeation chromatography (GPC) with refractive index (RI) detection and nuclear magnetic resonance spectroscopy (NMR). Each technology provides complementary information about a sample, such as average molecular weight, molecular weight distribution, monomer units, and end group composition.
These chemical properties (composition and mass parameters) are measured because they have an effect on the physical properties of polymers, and therefore their use in various applications. These traditional techniques cannot be used to determine 3D structure, which is greatly influenced by the flexibility of the polymer chain. It is predicted that the 3D structure of a polymer will have functional importance as synthetic polymers become increasingly sophisticated.1, 2 There is a close relationship between structural architecture and macroscopic properties.
The demand to accurately characterize this new functionality is likely to rise as polymers are increasingly used in highly regulated industries. Applications such as food contact materials and cosmetics are already attracting the attention of regulatory bodies.1
This application note demonstrates how a polymer can be differentiated and characterized using Ion Mobility Spectrometry-Mass Spectrometry (IMS-MS) based on its flexibility and structure. The technique is rapid and requires very little sample preparation.
The copolymers were first dissolved in 50:50 acetonitrile:water before further dilution to produce the following:
10 ppm PEG-r-PPG in 50:50 acetonitrile:water.
10 ppm PEG-b-PPG-b-PEG in 50:50 acetonitrile:water.
The polylactide sample was first dissolved in acetonitrile before further dilution to produce the following:
200 ppm polylactide and 20 ppm sodium iodide in acetonitrile .
|
MS system: |
SYNAPT G2 HDMS |
|
Ionization mode: |
ESI positive |
|
Infusion rate: |
10 μL/min |
|
Scan time: |
1 sec |
|
Extraction cone: |
5.0 V |
|
Desolvation temp.: |
200 °C |
|
Cone gas: |
Nitrogen, 20 L/hr |
|
Desolvation gas: |
Nitrogen, 600 L/hr |
|
Sample |
Capillary(kV) |
Sample cone(V) |
Source temp(°C) |
|---|---|---|---|
|
PEG-b-PPG-b-PEG |
2.5 |
100 |
120 |
|
PEG-r-PPG |
2.5 |
100 |
120 |
|
Polylactide |
3.1 |
50 |
80 |
Waters SYNAPT G2 HDMS is an orthogonal acceleration quadrupole Time-of- Flight (ToF) mass spectrometer with an integrated Triwave device that is capable of differentiating ions using mobility separation. Ions are guided from the ion source through the quadrupole to the mobility cell, and finally the ToF analyzer. The order of the technology within the instrument allows true MS/MS analysis to be carried out, if required, before ions are separated in the T-Wave ion mobility separation region according to their size, shape, and charge state. Finally, the ToF analyzer measures the mass-to-charge ratio of the separated ions.
When polymers are analyzed by mass spectrometry, generally the analyst is looking for a series of ions in the data that are caused by the polymer increasing in mass due the addition of monomer units. This gives a polymeric ion distribution. When mobility separation is also performed we look for a series of ions in a 3D data set. Figure 1 shows the polymer structures analyzed as part of this study.
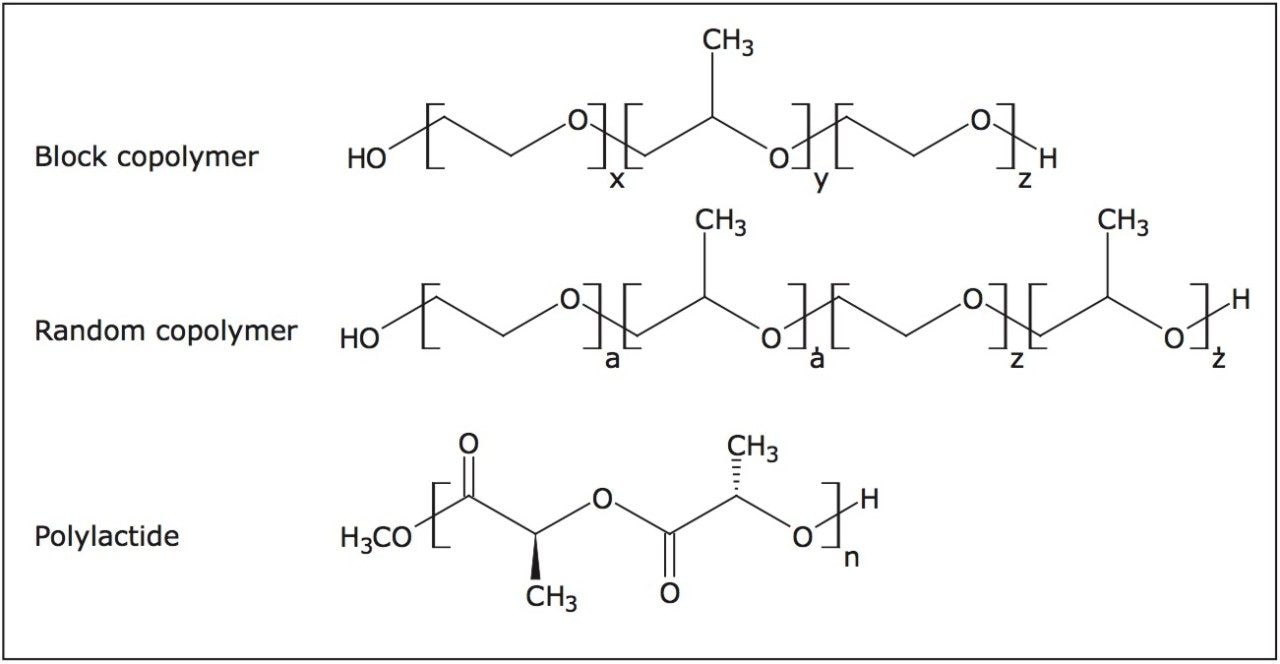
Figure 2 shows two mobility plots. The mass to charge ratio is on the x-axis, drift time on the y-axis, and ion intensity is represented by color. Both samples are copolymers containing PEG and PPG repeat units with an average molecular weight of approximately 2000 Da. Figure 2a presents the IMS data obtained for the block copolymer ions, the area with the highest ion intensity runs roughly diagonally across the plot. Figure 2b presents the IMS data when the random copolymer ions are analyzed. In the copolymer far greater bends, or kinks, are observed in the ion series.
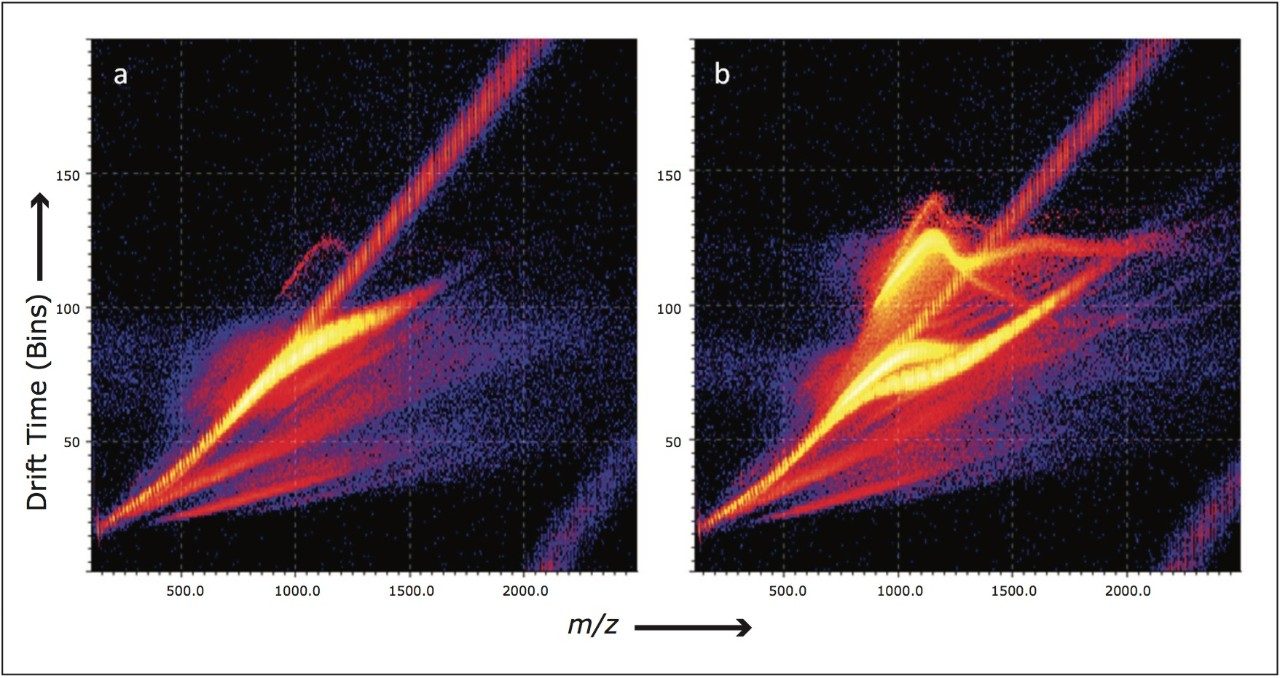
If we observe a roughly straight diagonal line in the mobility plots, this tells us that as the polymer increases in mass, there is a predictable relationship with its collision cross section area. A bend, or a kink, in the ion series indicates that as the polymer increases in mass, the 3D arrangement of the polymer chain changes and possibly folds back on itself which is ultimately dictated by the cationizing species(s).2
A simple comparison of both spectra shown in Figure 2 allows us to very quickly differentiate between the random and block copolymer. The random copolymer has many more isomers, therefore it is reasonable to expect more conformers for a given m/z with a variety of shapes and sizes. With some copolymers it may even be possible to use the mobility separation to isolate a series of related ions.
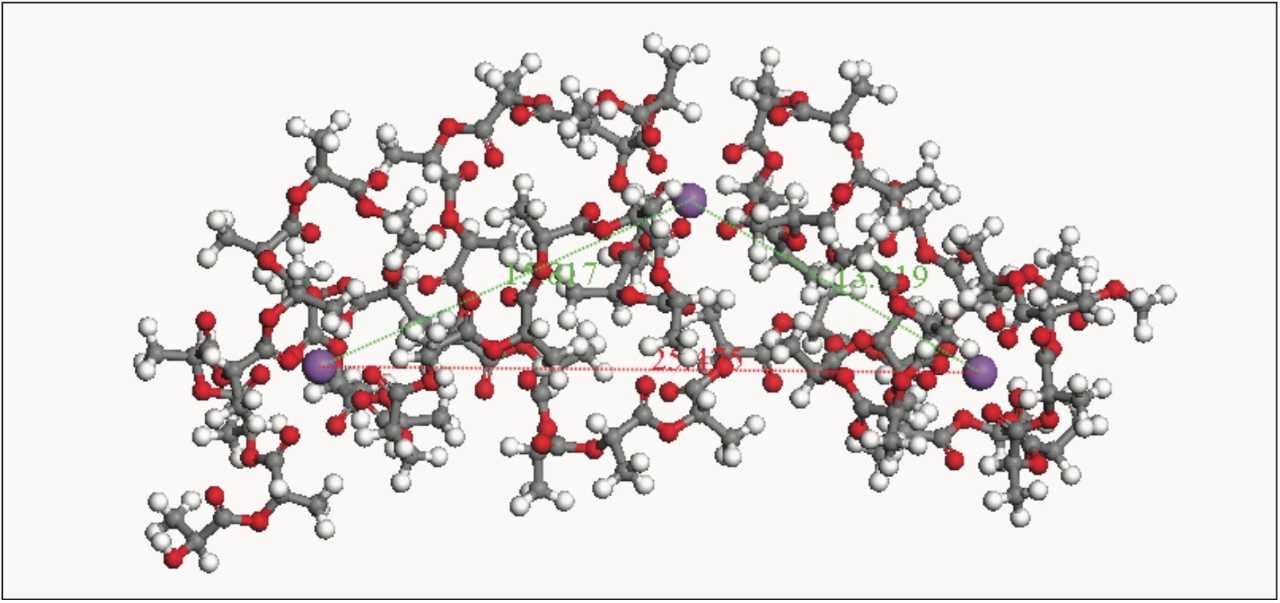
Recently, academic research has been carried out on ionized polylactides in the gas phase. The aim of this work was to establish the presence of folding of multiply charged ions and the degree of polymerization at which the folding occurs, for given charge states. Figure 3 shows a graph from Chemistry A European Journal.3 The authors produced both theoretical and experimental collision cross section areas for doubly and triply sodiated polylactide. The experimental values were obtained using a linear drift tube (University of Lyon, Dr. Ph. Dugourd).
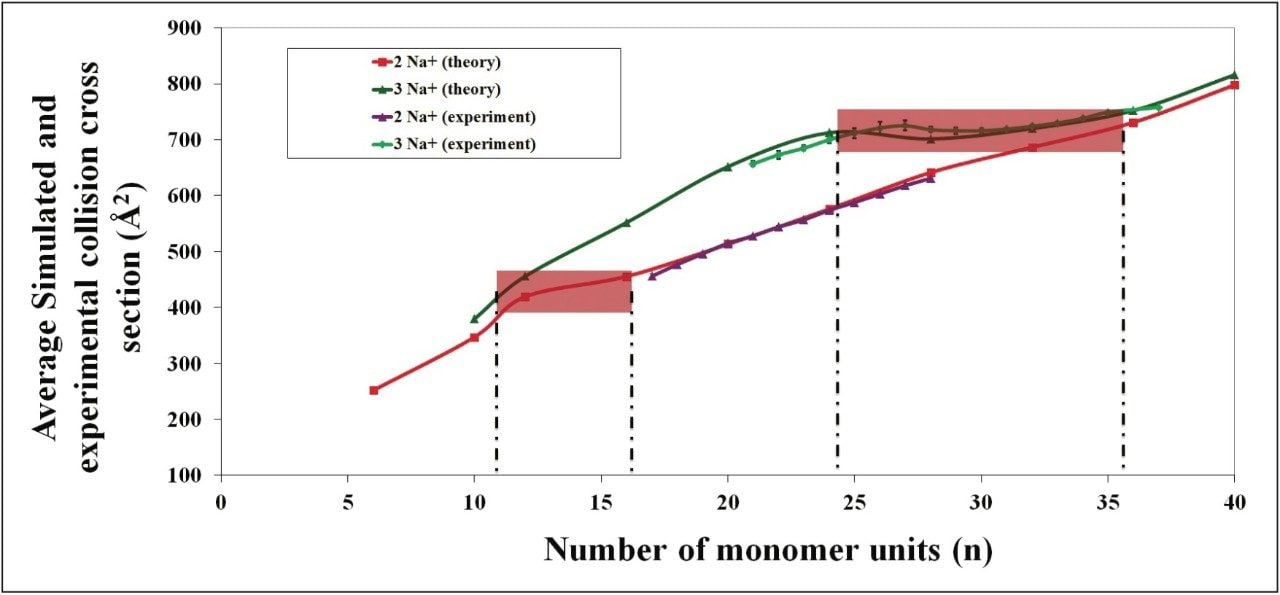
The authors determined that doubly charged polylactide folds between 12 and 16 monomer units, and between 24 and 36 monomer units when triply charged. This information was confirmed by the theoretical 3D structures for these polymer ions. Figure 4 shows a snapshot for the 3D calculated structure of the sodiated 28-mer triply charged polylactide.
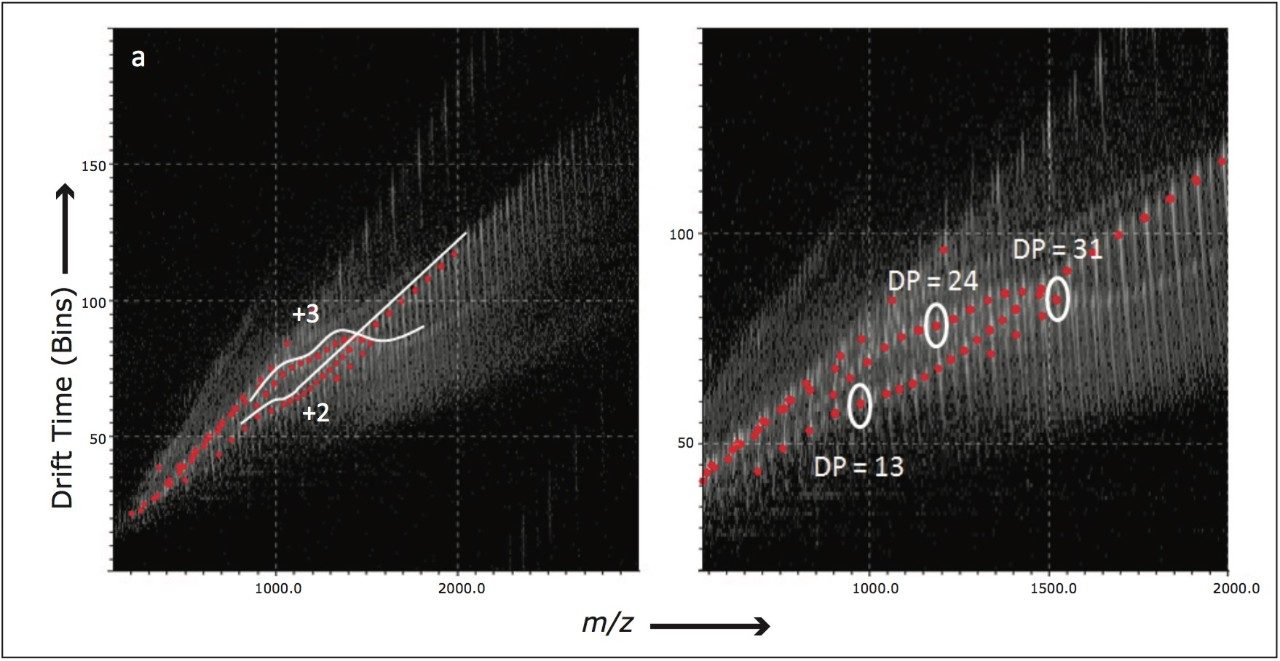
Similar experiments were performed using SYNAPT G2 HDMS with ion mobility functionality enabled. Figure 5 a shows the full mobility plot with the charge state of the two main ion series labeled. Figure 5 b is a zoomed image of the area of interest. Three ions of particular interest have been highlighted and labeled with their degree of polymerization (DP). These are the ions where the significant folding occurs and this is consistent with the published research performed on a linear drift tube.3 The increased sensitivity of the SYNAPT G2 HDMS provided additional information allowing the identification of two successive folding patterns for the triply charged ions.
A selection of polymers and copolymers were analyzed on the SYNAPT G2 HDMS with ion mobility enabled. The ions were separated according to their size, shape, and mass to charge ratio. This information can be used to characterize a polymer’s flexibility and 3D structure, measurements that cannot be made by traditional techniques or other commercially available mass spectrometers.
720004646, March 2013