
This is an Application Brief and does not contain a detailed Experimental section.
This application brief discusses successful identification of isobaric glycoforms (glycopeptides differing only in the stereochemistry of their glycans) in a tryptic digest of a glycoprotein using a proteomics workflow.
The ion mobility capabilities of SYNAPT G2 MS allows for observation of isomeric forms of a glycan at particular glycosylation sites.
Glycosylation is an important post-translational modification of proteins involved in processes, such as receptor recognition, protein solubilization, serum half-life regulation, and conformation stabilization. Differences in glyclosylation of particular proteins have been found to be indicators for certain cancers. Many biopharmaceutical proteins require glycosylation to be therapeutically safe and effective, and it is necessary to characterize and control this process. Often more than one glycan can be found at a given glycosylation site on a protein. When these glycans differ in their overall carbohydrate composition, the resulting glycoforms have different masses, and usually can be separated by LC. There are cases, however, where glycoforms differ not in their carbohydrate composition, but in how the glycan structure is assembled. These stereoisomeric structures all have the same mass; hence they are referred to as isobaric glycoforms. As such, they cannot be distinguished by mass, and also often not separated by LC. However, changing the stereochemistry of the glycan may alter the shape of the glycopeptide in the gas phase sufficiently to change its collisional cross section, thus making separation by ion mobility spectrometry possible. The speed and resolution of the ion mobility module of the SYNAPT G2 MS System enables such a separation to take place in a millisecond timeframe and therefore is fully compatible with LC separations during the course of a proteomics workflow, such as High Definition MSE (HDMSE).
Bovine Fetuin was reduced, alkylated, and trypsin digested with the aid of RapiGest surfactant following recommended procedures. The resulting peptide and glycopeptide mixture was analyzed using a linear acetonitrile gradient on a nano LC (nanoACQUITY UPLC System) and HDMSE on a SYNAPT G2 Mass Spectrometer. The data were analyzed with DriftScope, ProteinLynx GlobalSERVER (PLGS), and Spotfire Decision Site (TIBCO). Glycopeptide ions having identical m/z and retention times but different ion mobility drift times were deemed to be isomeric glycopeptide structures.
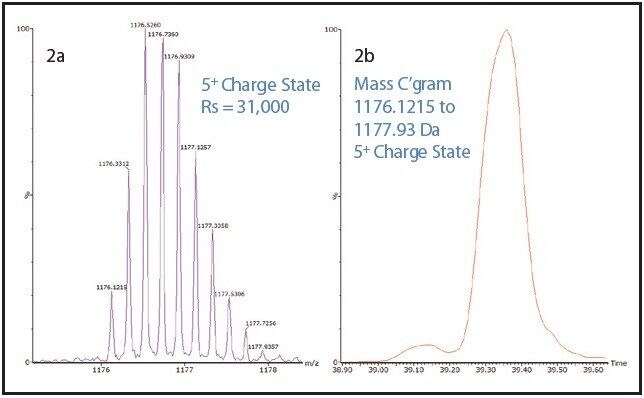
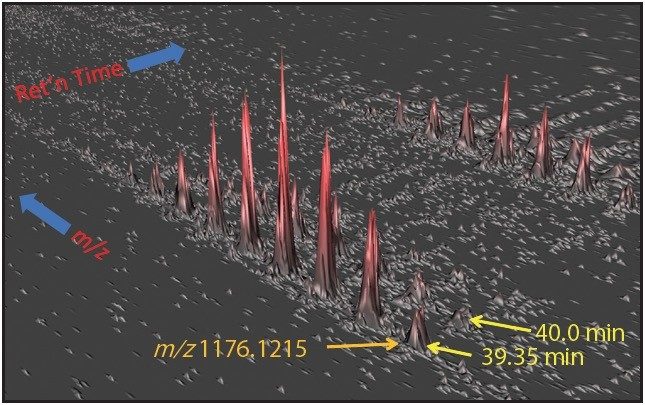
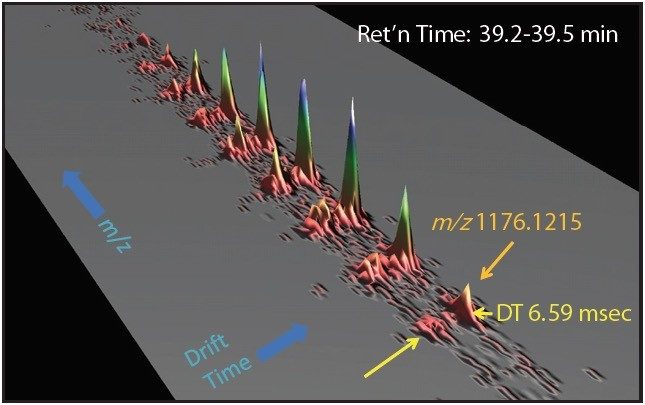
One such example is the 5+ charge state for the glycosylated peptide RPTGEVYDIEDTLETTCHVLDPTPLANCSVR, whose glycan has the composition 5 hexoses, 4 Nacetylhexosamines and 2 sialic acids, consistent with a biantenary complex carbohydrate. Figure 2A shows the mass spectrum for the ions that comprise this charge state, and Figure 2B is an extracted mass chromatogram for the ion cluster, with both appearing to be homogenous and representative of a single entity. This is reinforced by Figure 3, a three-dimensional display of the same ion cluster as Figure 2A. However, when this isotope cluster is viewed by m/z and drift time, (as shown in Figure 4) it can be clearly seen to separate into two ion clusters of identical retention time and m/z values but different drift times, the criteria for isobaric glycoforms. Further confirmation is shown in Figure 5, where following processing by PLGS, the data points for the two ion clusters are displayed in a cube plot that represent values for ion m/z, retention time, and drift time. Calculated retention times and masses differ by negligible values (0.02 min and 0.6 ppm, respectively), while there is a cluster drift time difference of 0.87 milliseconds.
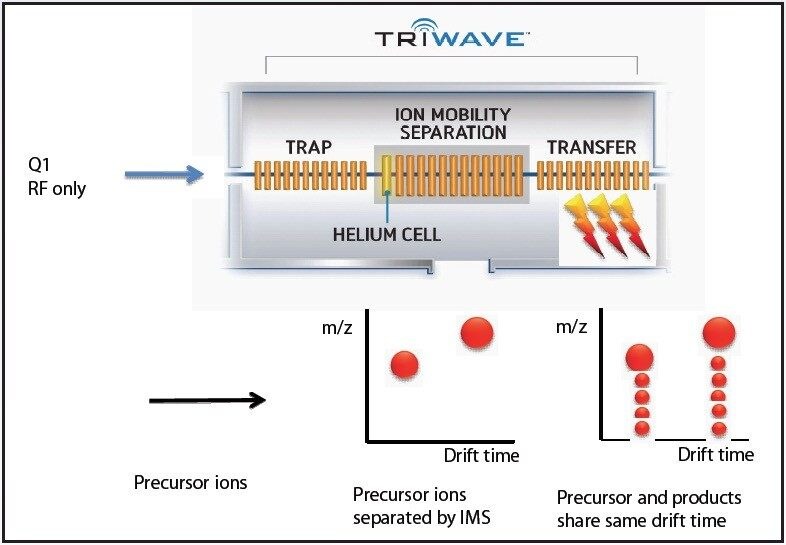
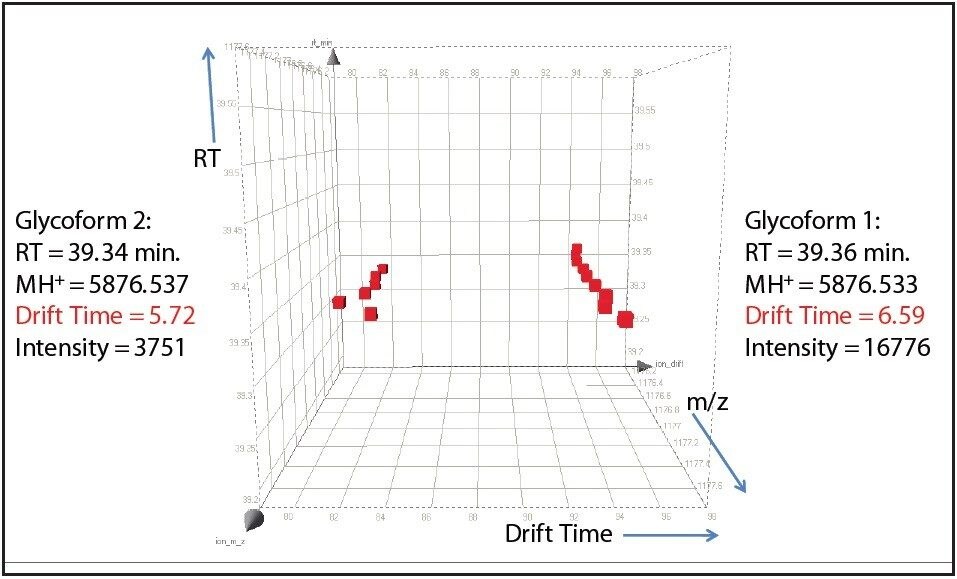
In order to fully understand the glycosylation of a protein, the existence of isomeric forms of a glycan at a particular glycosylation site must be observed if present. Such subtle differences could easily be overlooked by ordinary methods of analysis. The addition of ion mobility separation to LC-MSE data collection with the SYNAPT G2 MS, along with its enhanced mass resolution, make this instrument a unique tool for the study of protein glycosylation.
720003640, July 2010