Monitoring Amino Acid and Bispecific Antibody Homodimer by LC-MS for an Accelerated Upstream Process Development using Sartorius Ambr®15 Microbioreactor
Abstract
This application note details the use of the BioAccord™ LC-MS System for at-line process analytical technology (PAT) in upstream development, specifically targeting mispaired bispecific antibody (BsAb) homodimer monitoring. A series of cell culture conditions including media, downshifted temperature, and feed strategy were investigated in a Design of Experiments (DoE) manner. The experiments were processed on a Sartorius Ambr®15 high throughput microbioreactor system and samples were collected on different days for amino acid quantification and intact mass analysis. The waters_connect™ platform enabled a rapid and efficient workflow, streamlining sample analysis to expedite process development and enhance cell culture understanding.
Benefits
- Simplified and accelerated upstream process development of bispecific antibody cell cultures in high throughput microbioreactor systems by monitoring the intact drug substance and amino acids in a one-platform LC-MS System
- Streamlined and straightforward LC-MS acquisition and data processing using the BioAccord LC-MS System designed for ease of operation for analysts of all levels of expertise
- Design-of-Experiments (DoE) and multivariate regression analysis of the data revealed correlation between specific amino acids and homodimer formation in bispecific antibody cell cultures
Introduction
Bispecific antibodies (BsAb) could achieve better therapeutic effects by simultaneously binding two distinct targets or epitopes, outperforming monoclonal antibodies in more precise regulation of biological effects. However, BsAb production is complicated due to the formation of unwanted homodimers, which compromises purity and yield. Minimizing homodimer presence in cell culture supernatant is crucial for maximizing BsAb expression and cost-saving. This application note presents a comprehensive exploration of cell culture conditions for homodimer content optimization conducted on the Sartorius Ambr®15 high throughput microbioreactor system, and efficient homodimer monitoring realized using the BioAccord LC-MS System.
Experimental
Sample Preparation
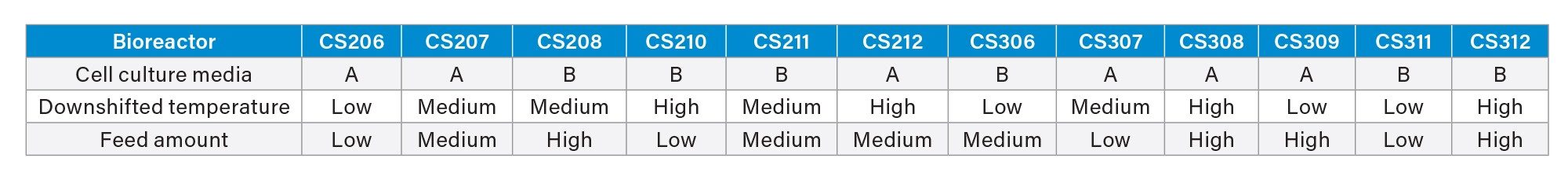
To reduce homodimer levels, a DoE experiment focusing on culture media, downshifted temperature, and feed strategy was designed (Table 1). A CHO-K1 fed batch process was employed for the experiment with 12 culture conditions on an Ambr® 15 microbioreactor system over 14 days. Samples were collected on Day 11, 13, and 14, processed with Protein A purification, deglycosylated by PNGase F, and analyzed for intact mass on a BioAccord LC-MS System. Amino acid samples were taken on Day 9–12 and 14, using analysis method previously described1 and the sample preparation method prior to analysis was slightly modified.1 Briefly, culture media were centrifuged and proteins were precipitated with acetonitrile. The supernatant was diluted at a 1:2000 ratio using 0.1% formic acid (FA) containing 3-chloro-L-tyrosine as internal standard for LC-MS analysis on a BioAccord System.
LC-MS Conditions for Intact Protein Analysis
|
LC-MS system: |
BioAccord LC-MS System with ACQUITY™ Premier BSM |
|
Ionization mode: |
Full scan |
|
Mass range: |
High (400–7000 m/z) |
|
Polarity: |
Positive |
|
Capillary voltage: |
1.5 kV |
|
Cone voltage: |
70 V |
|
Desolvation temperature: |
550 ℃ |
|
Intelligent data capture: |
Off |
|
Lockmass correction mode: |
Standard |
|
LC-MS software: |
waters_connect 3.1 or higher |
LC-MS Conditions for Amino Acid Quantification
|
LC-MS system: |
BioAccord LC-MS System with ACQUITY Premier BSM |
|
Column(s) |
ACQUITY Premier HSS T3 Column 1.8 μm, 2.1 x 150 mm (p/n: 186009469) |
|
Column temperature: |
40 ℃ |
|
Sample temperature: |
8 ℃ |
|
Injection volume: |
1 μL |
|
Flow rate: |
0.25 mL/min |
|
Mobile phase A: |
0.1% FA in H2O |
|
Mobile phase B: |
90% ACN/10% IPA/0.1% FA |
|
Ionization mode: |
Full scan with fragmentation |
|
Acquisition range: |
Small molecules (50–800 m/z) |
|
Polarity: |
Positive |
|
Capillary voltage: |
1 kV |
|
Cone voltage: |
20 V |
|
Fragmentation cone voltage: |
40–60 V |
|
Scan rate: |
5 Hz |
|
Desolvation temperature: |
550 ℃ |
|
Intelligent data capture: |
On |
|
Lockmass correction mode: |
Standard |
|
Acquisition time window: |
Start time = 0 minutes, End time = 14 minutes |
|
MS event table: |
0 minutes divert to waste, 0.8 minutes divert to MS, and 14 minutes divert to waste |
|
LC-MS software: |
3.1 or higher |
|
Informatics: |
UNIFI™ – Accurate mass screening using the cell culture media screening workflow |
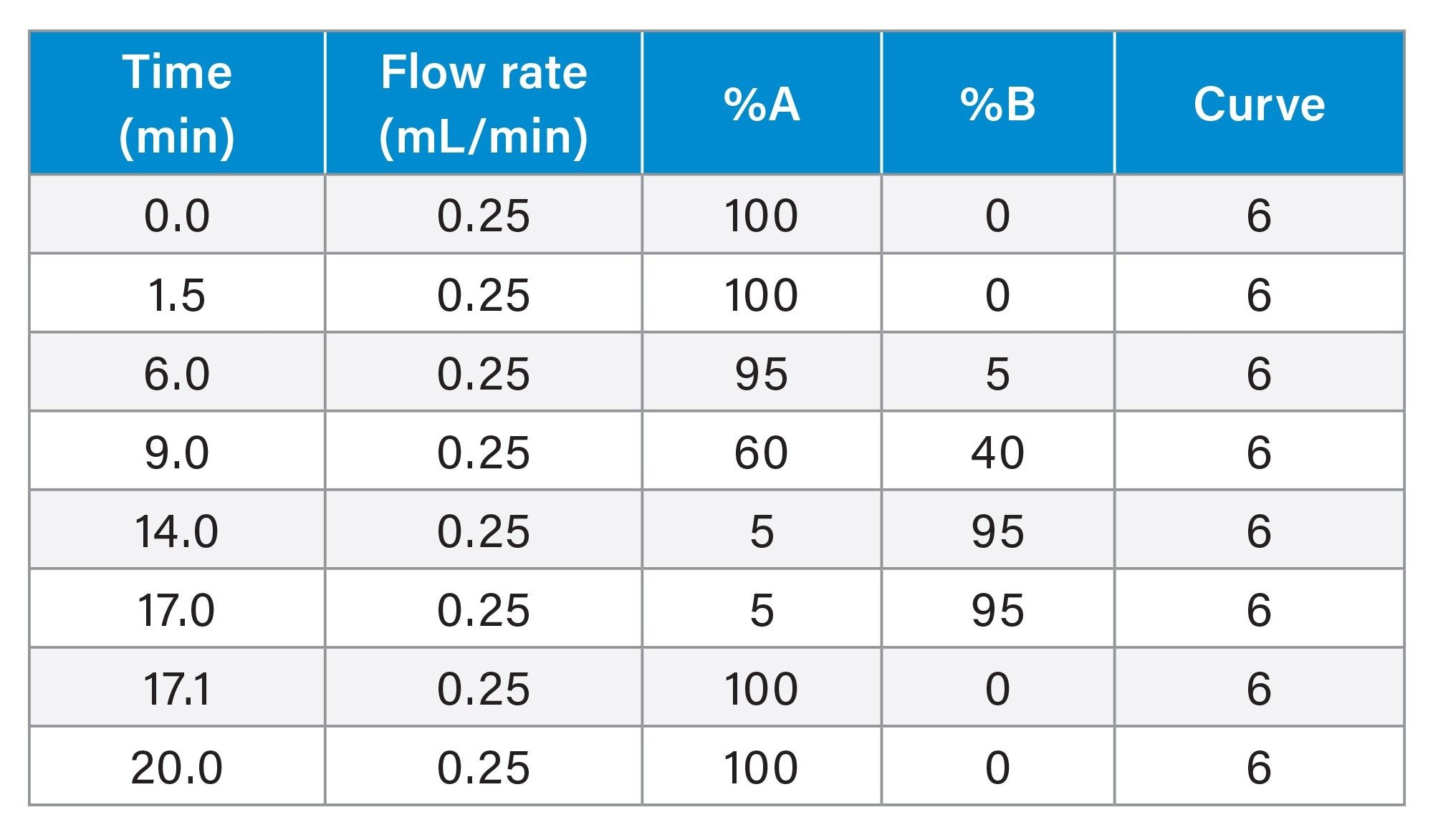
Gradient table
Results and Discussion
Determination of Relative BsAb Homodimer Content after Protein A Purification
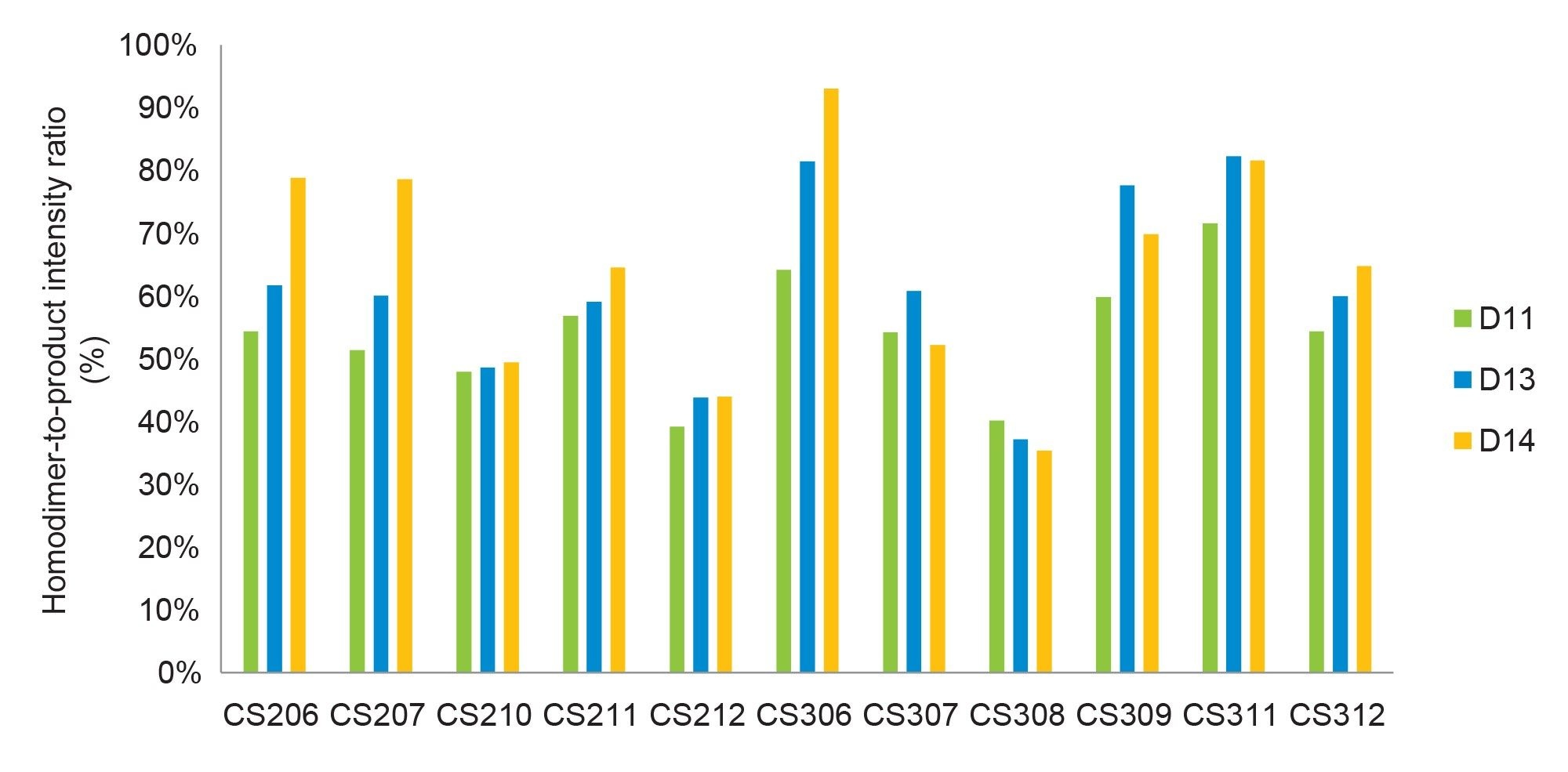
Biopharmaceutical companies typically employ a suite of analytical methods for bispecific antibody characterization, yet few are immediately suitable for differentiating products from homodimers during early process development. The platform LC-MS method, however, can distinguish between the two without extensive method development. In this study, we utilized the intact mass analysis approach to assess homodimer levels under various early-stage cell culture conditions. After Protein A purification, samples were analyzed using a BioAccord LC-MS System, which was capable of identifying both homodimers and products successfully. As a marginal 3 kDa molecular weight difference between the homodimer (148 kDa) and product (145 kDa), similar ionization efficiencies allow for fair comparison of signal intensities in MS between these two species. Consequently, the homodimer-to-product intensity ratio serves as a relative quantification method for homodimer content. Figure 1 presents a histogram depicting the homodimer-to-product intensity ratio under various cell culture conditions over time, revealing reduced homodimer levels in CS210, CS212, and CS308, which all have a high downshifted temperature.
Monitoring amino acids content changes among multiple cell culture conditions
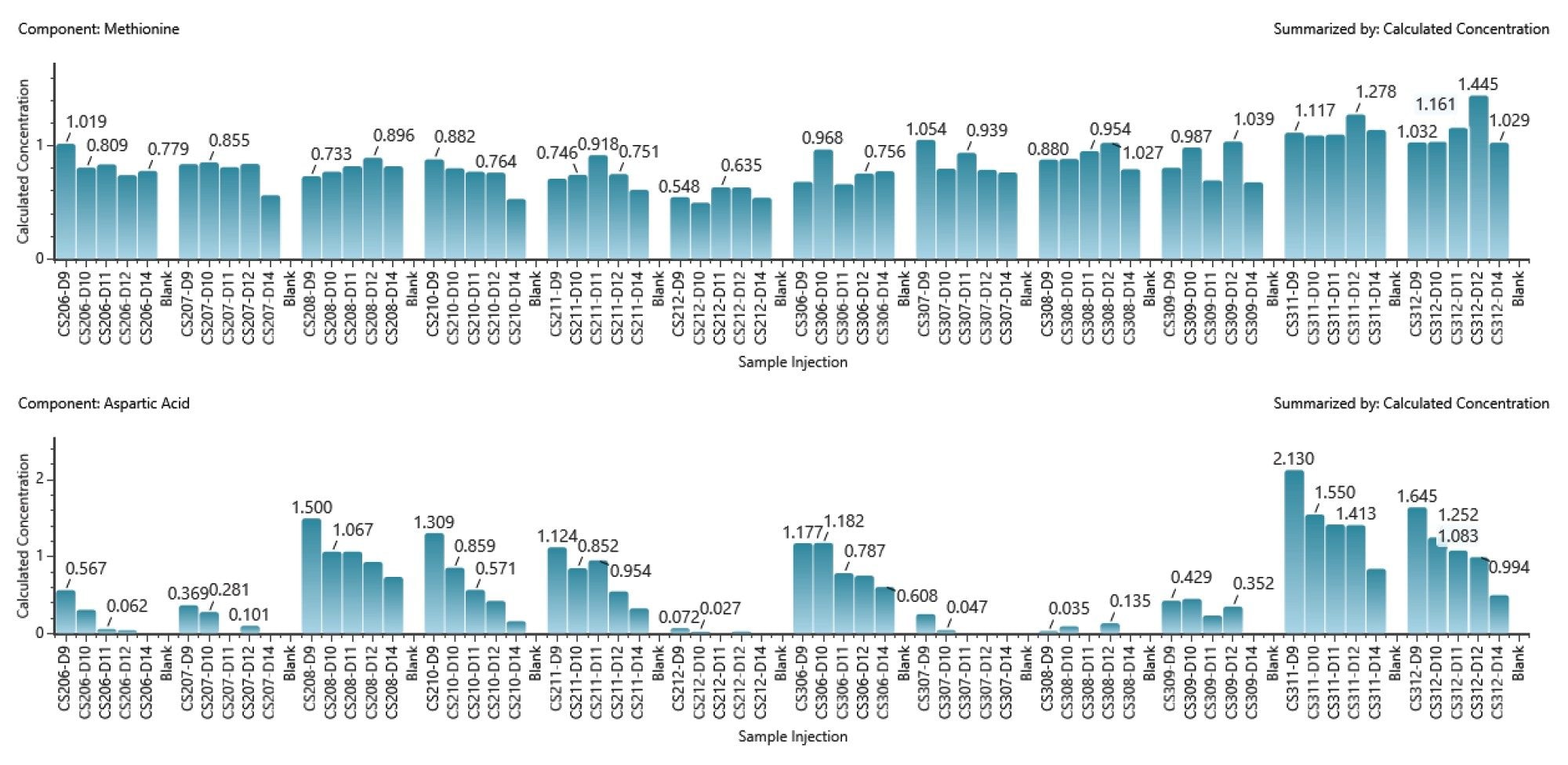
Optimizing cell culture media is a key aspect of upstream process development, in particular focusing on amino acid concentration monitoring considering their role as protein building blocks and metabolic intermediates, and hence significantly influencing bioproduct quality. Using a BioAccord LC-MS System, 20 amino acids were quantitatively analyzed, with the methodology detailed in a prior publication.1 Figure 2 illustrates the variation in amino acid concentrations across the DOE study over time, highlighting minor fluctuations for some, like methionine, and more pronounced changes for others, such as aspartic acid, under various cultivation conditions.
Multivariable Regression Analysis
All amino acid concentrations on Days 9–12 and 14, media type, downshifted temperature, and feed strategy were further analyzed by JMP to determine their correlation with homodimer ratios. Certain amino acids, designated as AA1 and AA2 here, were found to negatively correlate with homodimer content, whereas AA3 shows a positive correlation (Figure 3). In addition, downshifted temperature exhibits a weak negative correlation (Figure 4). No significant correlation was observed between homodimer content and other cell culture conditions (Figure 4).
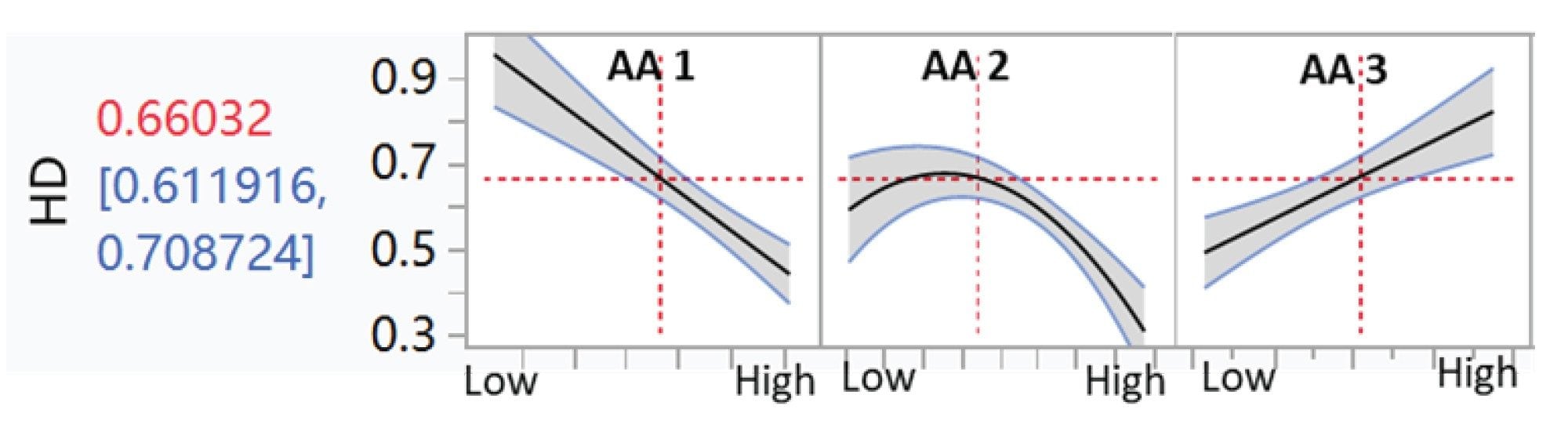
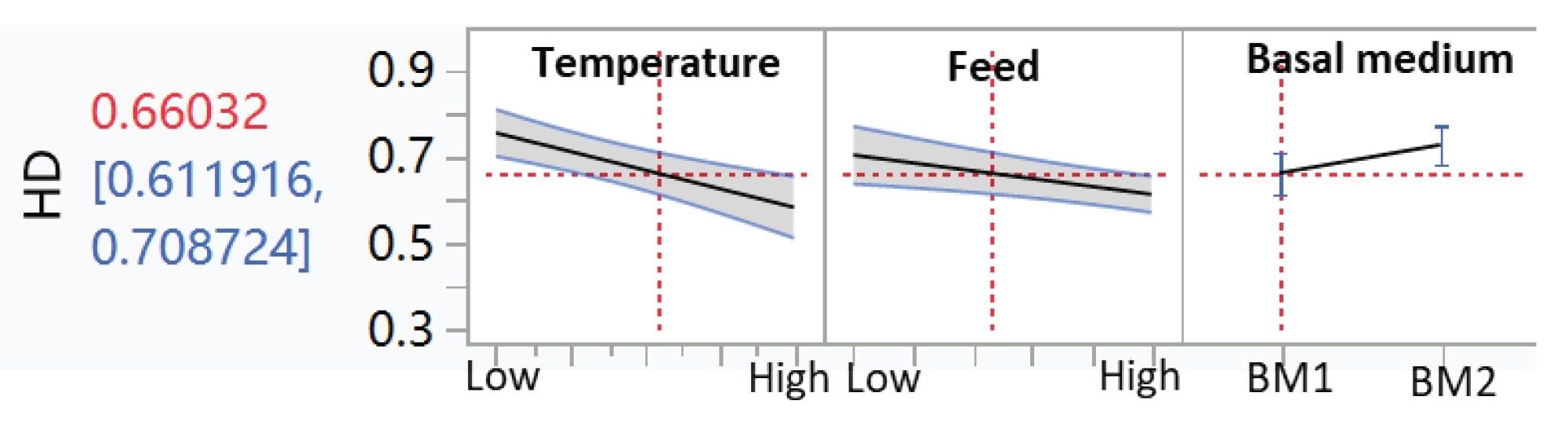
Conclusion
An upstream bispecific antibody process optimization by DoE study focusing on homodimer content was performed on a Sartorius Ambr® 15 microbioreactor system in conjunction with a BioAccord LC-MS System. The BioAccord LC-MS System enabled high-throughput analysis for intact mass analysis and amino acid quantification, for a large number of samples from the Ambr® 15 with 12 culture conditions over 14 days. Multivariable regression analysis revealed that three amino acids are strongly correlated with homodimer content, whereas downshifted temperature exhibits a weak negative correlation. Overall, the synergy between the BioAccord LC-MS System and the Ambr® 15 bioreactor significantly enhances the efficiency of upstream process development.
References
- YW Alelyunas, MD Wrona, YQ Yu. Quantification of Underivatized Amino Acids in Cell Culture Media Using the BioAccord™ LC-MS System, Waters Application Note, 720007766, October 2022.
720008343, May 2024