This application note demonstrates the use of MSE data acquisition for detecting reactive metabolites using clozapine as a model compound. Through integrated charge state deconvolution and detection using the Metabolite Identification Application Solution in UNIFI, all metabolites, including parent, phase I metabolites, and phase II GSH conjugates are detected and tracked across the dataset automatically.
Screening for reactive metabolites is commonly carried out in pharmaceutical drug discovery/development as part of the risk assessment of a compound, or compound class. The reactive metabolite hypothesis implicates bioactivation of drug compounds and subsequent haptenization or inactivation of proteins with clinical toxicity. To that end, significant effort has been made towards reducing the propensity of candidate drugs to undergo metabolic activation.
One way that reactive metabolites can be assessed is through the use of in vitro incubations fortified with trapping reagents such as glutathione (GSH). The formation of reactive intermediates can be followed by detection and characterization of “trapped” glutathione adducts using a variety of LC-MS approaches. Popular methods for detecting GSH conjugates include monitoring the ESI+ signature GSH neutral loss of 129,1 ESI- precursor ion scanning for the 272 diagnostic product ion,2 or using 1:1 stable isotope labeled GSH and detection based on a signature isotope difference with similar peak intensity under ESI- conditions.3
In addition to understanding the rate and pathways of formation for glutathione-containing metabolites, information on parent compound is often desired to estimate conversion rate of the compound in the study. Another complication of glutathione adducts is that they often exist as multiple-charged species with detection based on positive electrospray ionization, the widely used ionization technique for the majority of parent compounds. A failure to detect metabolites at low levels, and low and/or variable response for precursor ions and their product ions, can result in false negatives.
High-resolution mass spectrometry (HRMS) approaches are becoming more popular as gathering detailed precursor and product ion information is possible. Software tools can then comprehensively interrogate full scan data for expected and difficult-to-predict reactive intermediates.
Since Waters introduced MSE based data acquisition, which provides simultaneous acquisition of full scan precursor and product ion data, it has been possible to perform a generic data acquisition and produce fragment ion information in a non-selective manor, thus simplifying data acquisition.4
This application note demonstrates the use of MSE data acquisition for detecting reactive metabolites using clozapine as a model compound. Through integrated charge state deconvolution and detection using the Metabolite Identification Application Solution in UNIFI, all metabolites, including parent, phase I metabolites, and phase II GSH conjugates are detected and tracked across the dataset automatically.
|
LC system: |
ACQUITY UPLC I-Class |
|
Column: |
ACQUITY UPLC BEH C18 1.7 μm, 2.1 x 100 mm |
|
Column Temp. : |
60 °C |
|
Sample Temp. : |
10 °C |
|
Injection Volume: |
10 μL |
|
Flow Rate: |
0.6 mL/min |
|
Mobile Phase A: |
H2O with 0.1% formic acid |
|
Mobile Phase B: |
Acetonitrile with 0.1 % formic acid |
|
Gradient: |
0-60% B in 6 min, 60-90% B in 2 min, held at 90% B for 1 min before returning to the initial condition. The total run time was 10 min. |
|
MS system: |
Xevo G2-S QTof |
|
Ionization mode: |
ESI+, resolution |
|
Experiment: |
MSE |
|
MSE settings: |
Low CE 2.0 eV, high CE Ramp 10-30 eV |
|
Acquisition range: |
50-1200 m/z |
|
Capillary voltage: |
1 V |
|
Cone voltage: |
30 kV |
|
Source temp.: |
120 °C |
|
Desolvation gas temp.: |
550 °C |
|
Cone gas flow: |
20L/h |
|
Desolvation gas flow: |
1000L/h |
|
Scan time: |
0.1 s |
UNIFI Scientific Information System
Clozapine with final concentration of 10 μM was incubated in human liver microsomes (1 mg/mL final protein concentration, 150 donor pool, BD Gentest) in the absence or presence of GSH (5 mM) and/or cytosol (1 mg/mL). After starting the reaction by NADPH addition, the reaction was followed at 37 °C for 2 hours with 100-μL aliquots withdrawn at 15-min intervals. For each withdrawn sample, 2 volumes of cold acetonitrile were added to quench the reaction. The quenched solution was then centrifuged for 20 min at 15,000 RPM and 10 °C to precipitate proteins. Finally, the supernatant was transferred to a 2-mL analytical vial and diluted with 1 volume of H2O.
Experiments were performed on an ACQUITY UPLC I-Class System and a Xevo G2-S QTof high resolution mass spectrometer, using the UNIFI Scientific Information System.
Clozapine, an antipsychotic agent, carries a black box warning for agranulocytosis, among other side effects. While clozapine alone does not exert any direct cytotoxicity in in vitro tests, it has been postulated that the nitrenium reactive intermediate formed either from clozapine or its phase I metabolites may play a possible role (Figure 1).5 Clozapine metabolites from both phase I and phase II biotransformation pathways have been extensively characterized by LC-MS and NMR6 through the use of trapping studies.
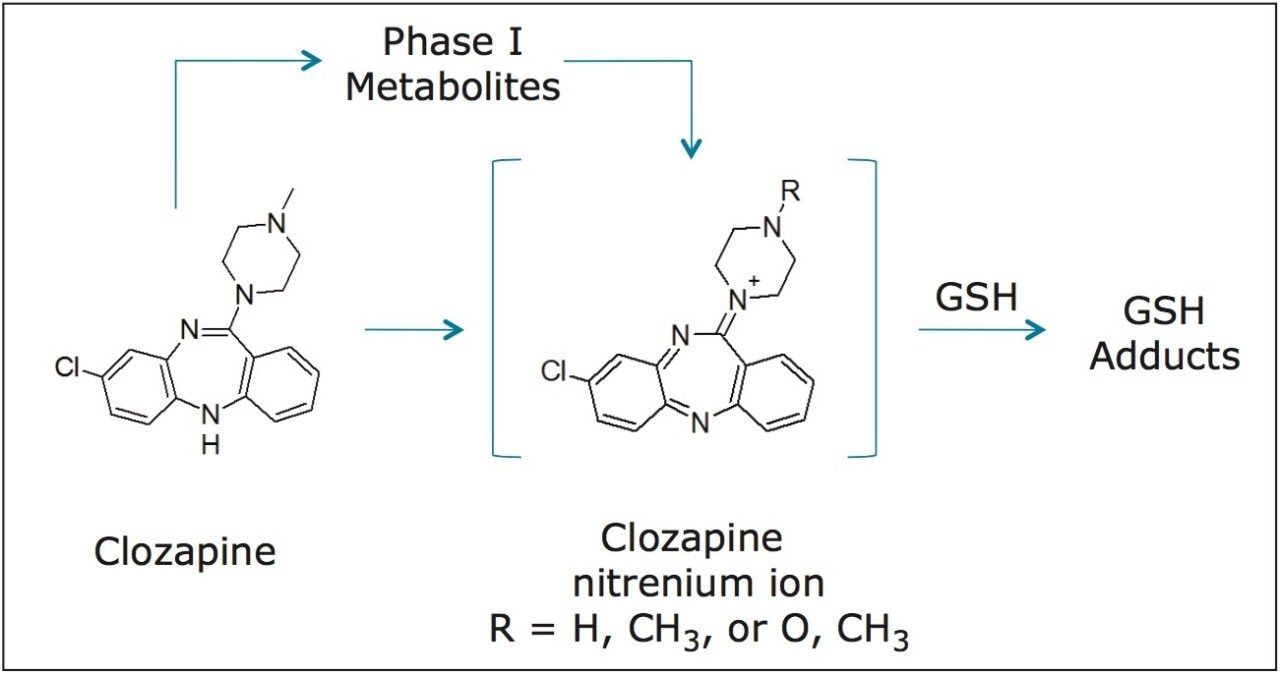
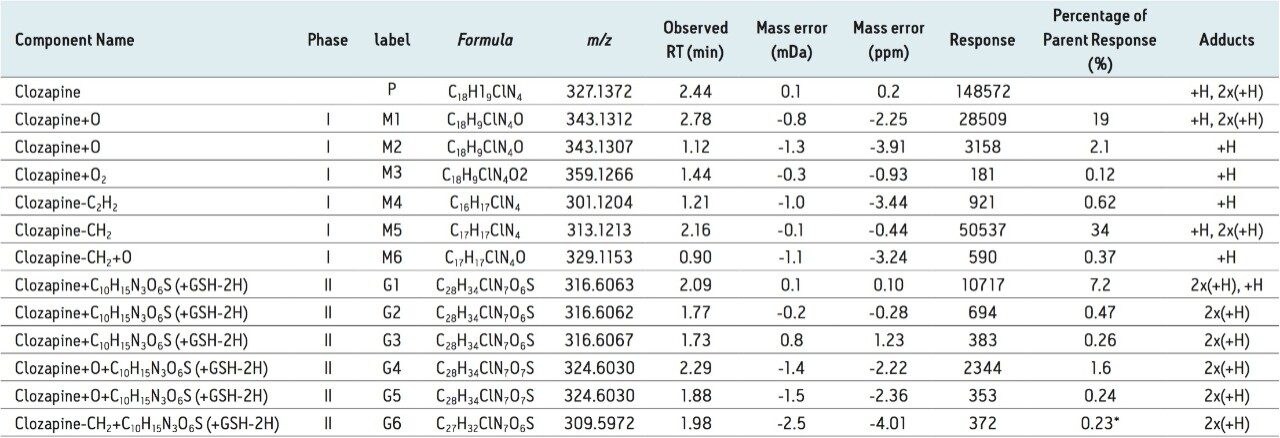
In the study, clozapine was incubated in human liver microsomes. Three conditions were tested: -GSH; +GSH; and +GSH +cytosol. Time points for each condition were collected at 15 min intervals over two hours. These samples were analyzed by LC-MS using MSE acquisition with positive electrospray ionization on the Xevo G2-S QTof MS platform under generic conditions. Data was processed using a generic set of processing conditions which searches for common phase 1 biotransformation pathways as summarized in Table 1.
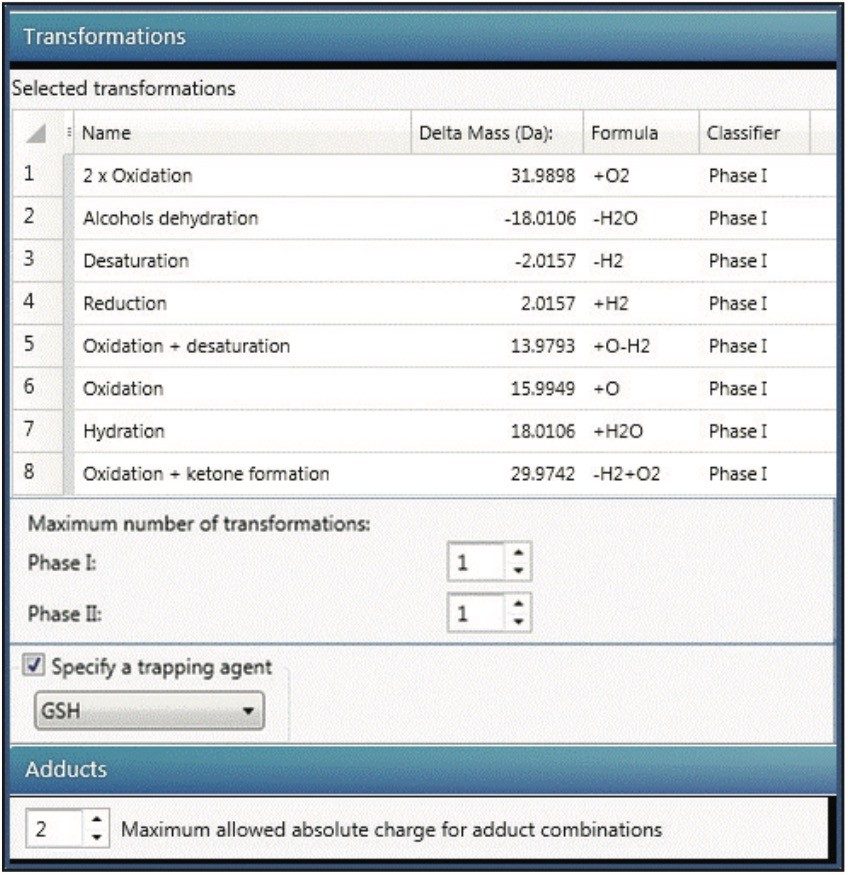
Identification of GSH adducts was enabled by checking the “Specify a trapping reagent” box in UNIFI Software and choosing GSH. For charge state deconvolution, 2 was chosen for the “Maximum allowed absolute charge for adduct combination” in the “Adduct” panel in data processing (Figure 2).
In UNIFI’s data review mode, a summarized XIC (extracted ion chromatogram) of all identified components is displayed for each sample/injection. Figure 2 shows data for the 90-min incubation containing GSH. Four major components are visible: clozapine, a +O metabolite, the -CH2 metabolite, and one +GSH-2H adduct at RT 2.1 min (Figure 3, top). Each component can be visualized either by choosing a specific XIC either by clicking on the peak in the summarized XIC, or by selecting a row in the component table where all identified metabolites are listed. For example, by choosing the +GSH-2H adduct peak at RT 2.1 min, a chromatogram is displayed showing, in addition to the major peak, two additional minor GSH adducts having the same mass (Figure 3, middle). Similarly, by choosing +O+GSH-2H metabolite in the component table, an extracted chromatogram is displayed showing one major and one minor metabolites with the same mass (Figure 3, bottom).
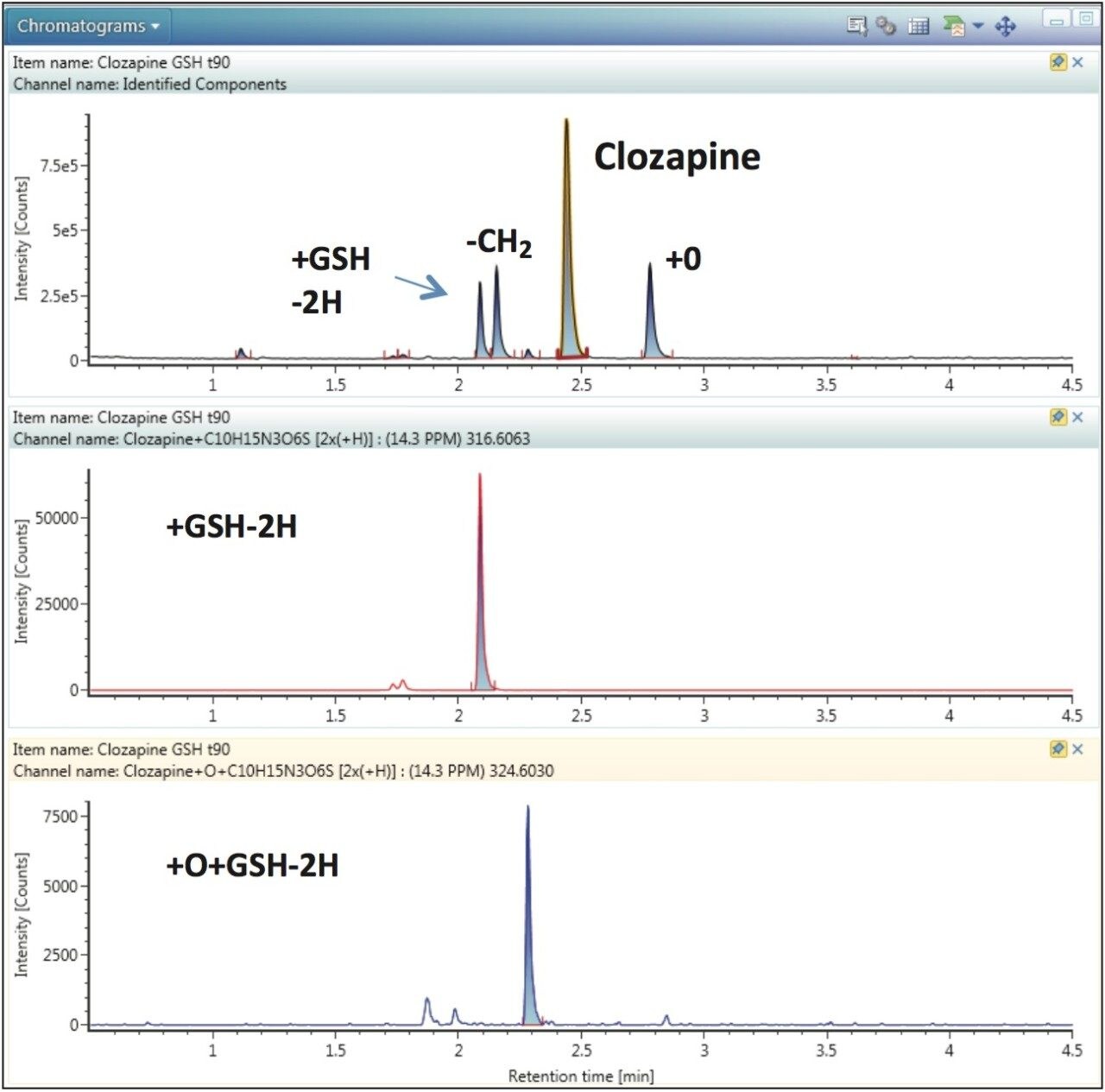
Table 1 summarizes data for the 12 metabolites observed in this study, including six phase I metabolites and six GSH adducts. The summary table also contains an “Adducts” column, where the observed charge state is displayed and listed in order of detected abundance. The column shows that for phase I metabolites, all compounds exist predominantly in a single-charge stage. For phase II metabolites, on the other hands, all the detected adducts are present exclusively in the doubly-charged state.
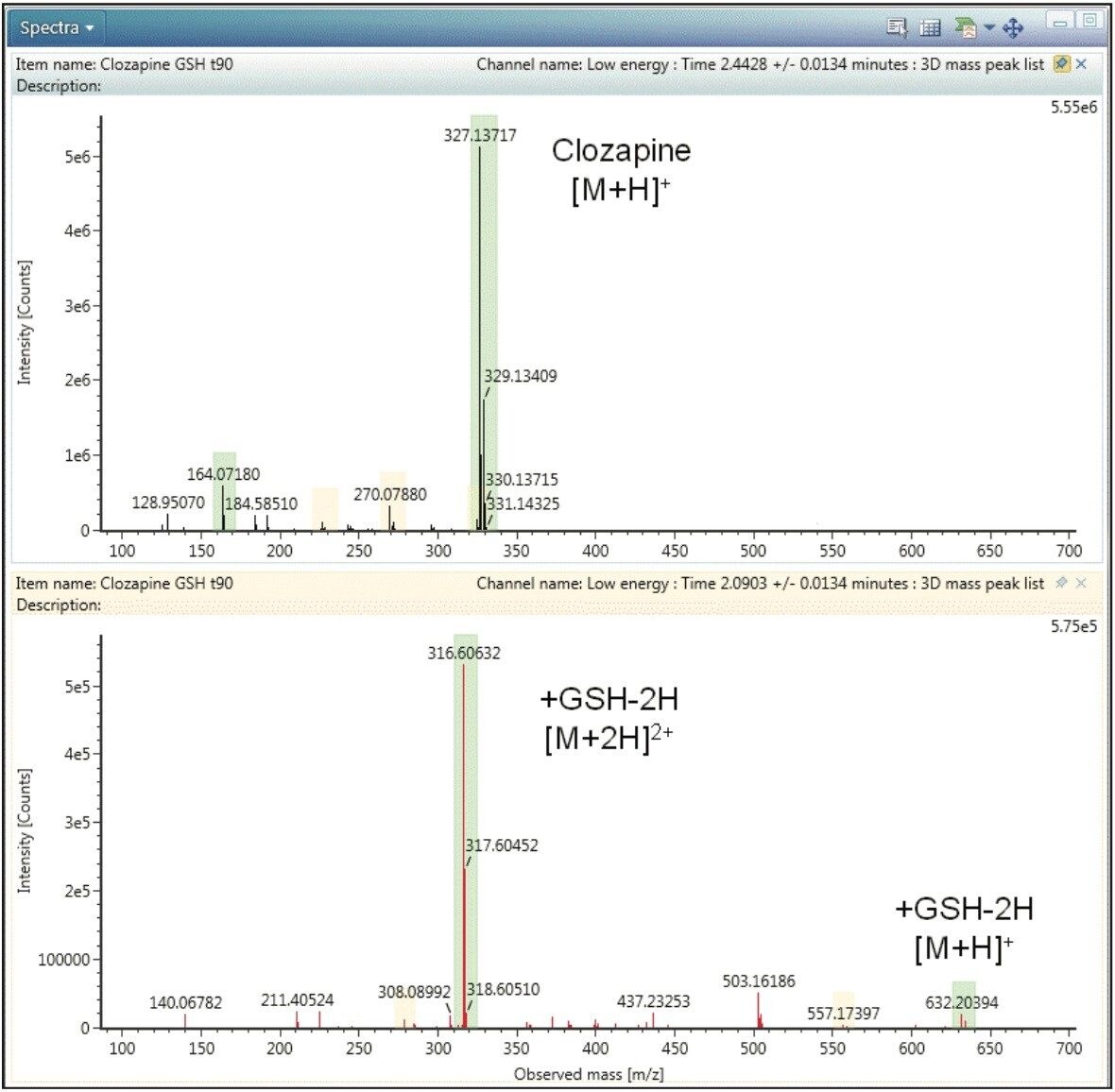
This typical behaviour is shown in Figure 4 in which clozapine is compared to the GSH adduct at RT 2.09 min, G1, as an example. In the spectrum of clozapine, the compound exists primarily as a singly charged species with an observed [M+H]+ = 327 (Figure 4, top), while the GSH adduct, G1, exists mostly as the doubly charged species [M+2H]2+ = 316.6 with only minor levels of the singly charged [M+H]+= 632.2 species (Figure 4, bottom). Less abundant GSH adducts were not observed as singly charged ions. Automatic deconvolution and grouping of all detected charge states (prior to data interpretation) is therefore essential for detecting GSH adduct under ESI+ conditions.
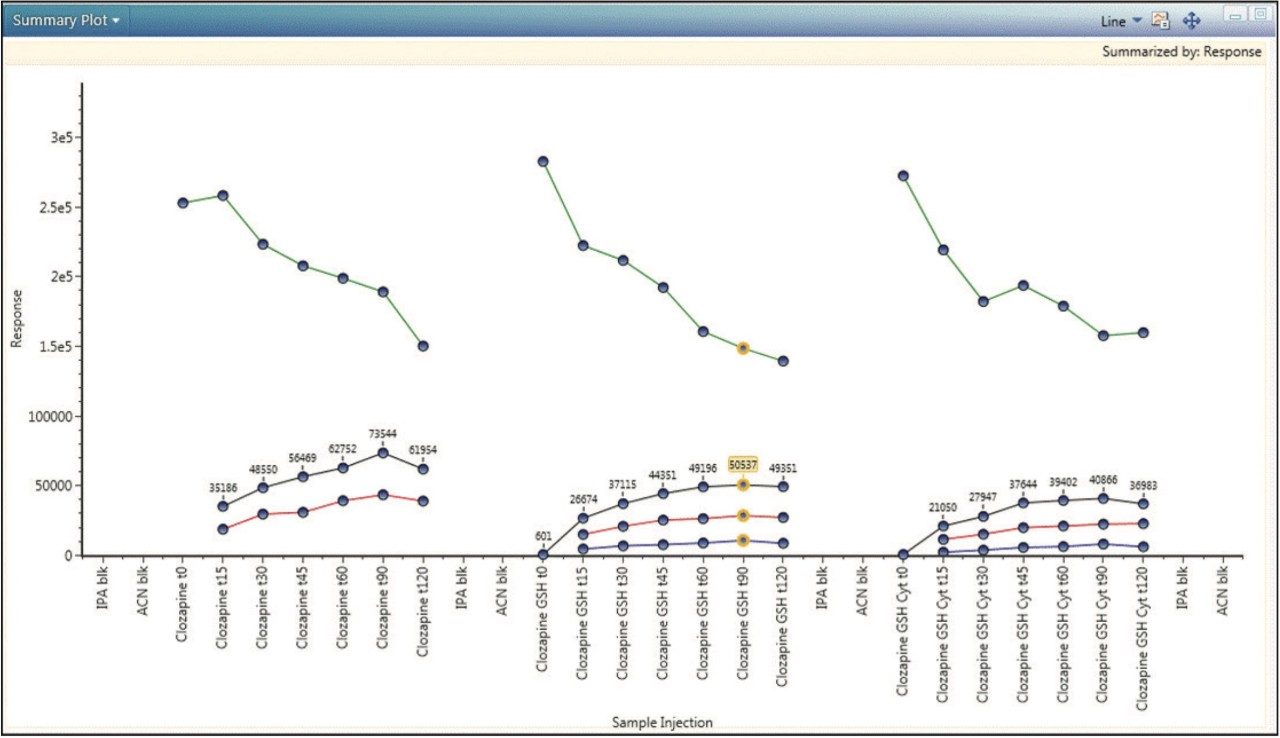
Finally, a complete picture of the study can be obtained by plotting observed components across all samples. Figure 5 is a summary plot for the response of clozapine and its major metabolites. The plot shows a decrease in clozapine concentration over time, with a corresponding increase in the concentrations of both phase I and phase II metabolites. Approximately 40% clozapine was metabolized at 90 minutes. The plot provides additional visual confirmation and supports positive identification of these metabolites.
A simple and generic method has been described for the simultaneous detection and quantitation of parent compound, phase I metabolites, and GSH adducts in a microsomal incubation, using MSE generic acquisition under positive electrospray ionization conditions. Under positive electrospray ionization conditions, glutathione adducts are typically present as multiple-charged species, resulting in no detection or low sensitivity based on singly charged precursor ion detection. The automatic data deconvolution and charge state summarization of UNIFI Software has provided the key functionality necessary for their detection under ESI+ conditions.
720004804, November 2013