This is an Application Brief and does not contain a detailed Experimental section.
This application brief demonstrates the advantages of collecting multiple reaction monitoring (MRM) and MS scan data in parallel (RADAR acquisition), in support of consumer protection legislation using Xevo TQ-S MS System with an APGC Source.
RADAR allows accurate quantification of target compounds, while at the same time, it acquires MS scan data to monitor matrix components or other compounds. This is accomplished with little or no impact on the MRM data.
Brominated flame retardants (BFRs) are compounds used to decrease the likelihood and intensity of fires. These compounds are present in a wide variety of consumer products, including electronics, clothes, and furniture. Due to the Restriction of Hazardous Substances (RoHS) Directive (2002/95/EC), these compounds are banned in various types of electronic equipment. This is also closely linked with the Waste Electrical and Electronic Equipment Directive (WEEE, 2002/96/EU), which is a legislative initiative to solve the problem of large amounts of toxic e-waste.
BFRs are most commonly analyzed by targeted GC-MS methods using single ion recording (SIR) or MRM. The problem with a purely targeted approach is that it ignores other related compounds or matrix background. The Waters Xevo TQ-S MS allows collection of MRM and MS scan data in parallel in one acquisition using RADAR.

The Xevo TQ-S MS System was coupled with a GC using an APGC Source and operated in RADAR mode. A suite of polybrominated diphenyl ethers (PBDEs) was analyzed with two MRM transitions chosen for each degree of bromination. An MS scan function with a mass range 50 to 1050 Da was simultaneously acquired. An extracted sample of a PC keyboard – produced before the July 2006 legislation banning BFRs – was analyzed for PBDEs.
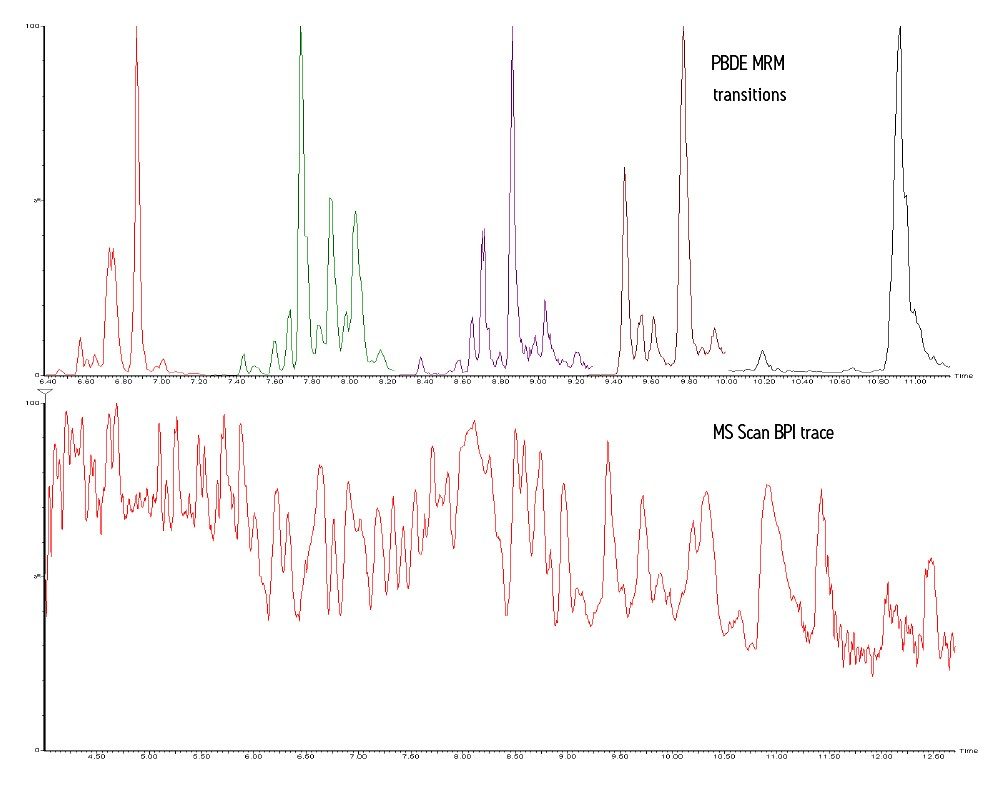
The acquired MRM transitions were used for quantification of PBDEs in the sample. The MS Scan function was interrogated for related compounds or possible interferences. Figure 1 shows an overlaid trace of the MRM transitions (upper chromatogram), along with a BPI trace (lower chromatogram) of the MS scan data. The MS scan in Figure 1 is intense and very complex. The cluster/strip function within MassLynx Software was used to extract pairs of ions with a separation of 2 Da, which highlighted spectra with halogenated isotope patterns. The NIST08 mass spectral library was used to find spectra of compounds suspected to be in a sample of this nature. Figure 2 shows the spectrum of the peak at 11.43 mins, with the molecular ion cluster magnified. Also, a comparison between the isotope cluster extracted from the suspect peak against the theoretical isotope model of C14H8Br6O2 is shown.
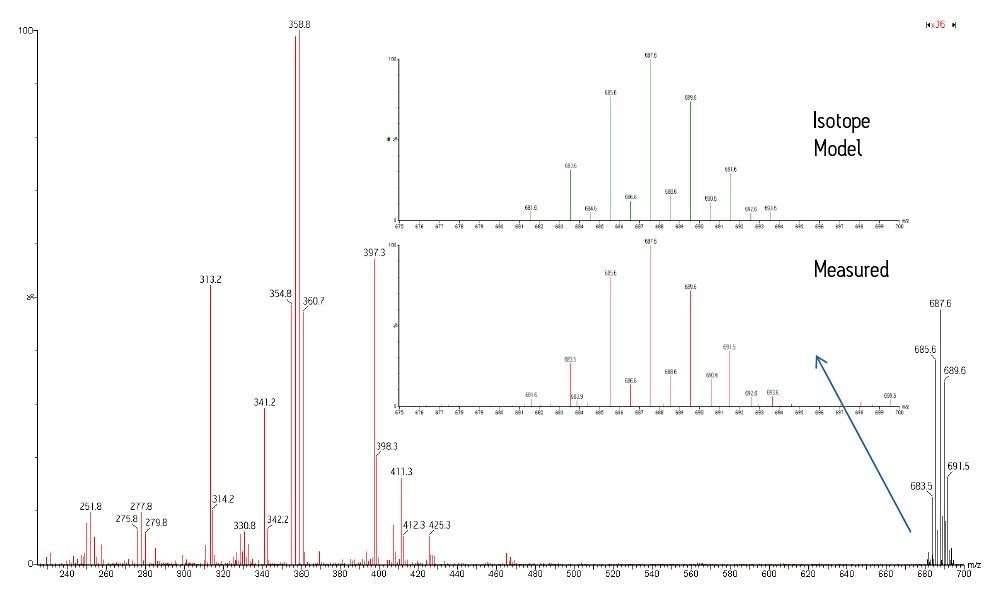
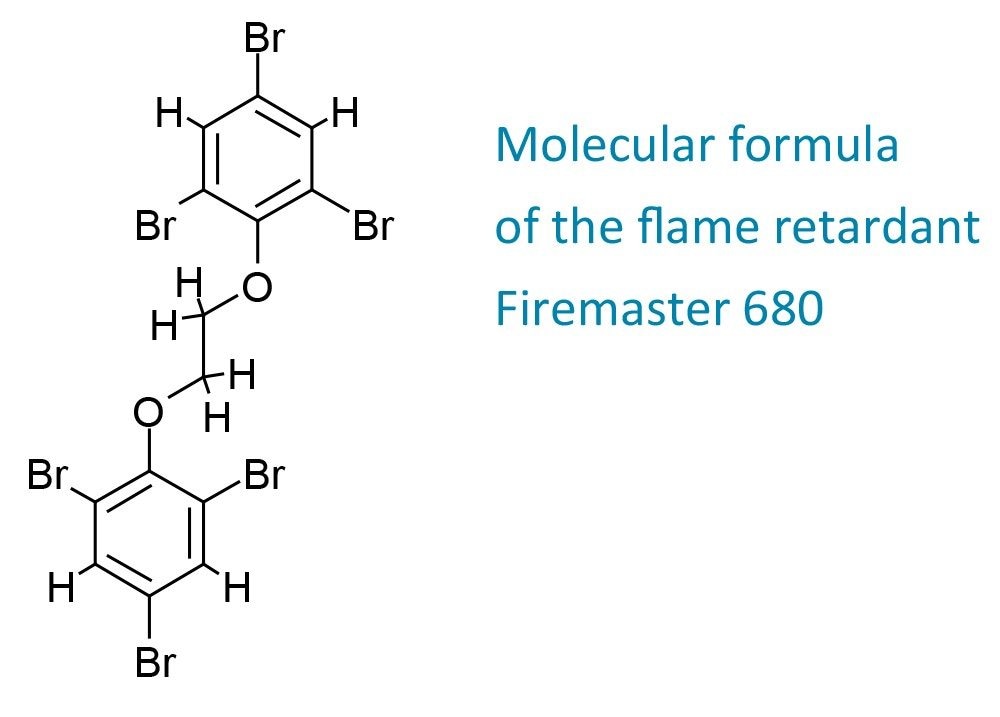
The proposed molecular formula is consistent with the compound tribromophenoxyethane, which is the active ingredient from a commercial product, FireMaster 680. Moreover, the fragment cluster (m/z 357 and 359) indicates dissociation (loss of C6H2Br3+CH3) of the molecular ion, which further supports this hypothesis. Further work is required to determine the identity of this compound with more confidence.
720004033, July 2011