
The aim of this application note is to evaluate the potential of fast polarity switching, resolution, and speed of analysis of pesticides in potatoes, oranges, and cereal-based baby food using UPLC-MS/MS. Additional pesticides with different polarities, transformation products, and structural isomers (butocarboxim sulfoxide and aldicarb sulfoxide) were included to extend the list of compounds studied.
The European Union residue monitoring program 2005–2007 establishes the need to cover 55 active ingredients in various foods, including potatoes, oranges, and baby foods.1 Twenty of these pesticides are suitable for multi-residue LC-MS analysis. The majority of this group has a positive polarity in electrospray mode and only one (fludioxonil) has a negative polarity, normally requiring two injections (one in each polarity ion mode). Consequently, compounds with negative polarity are often excluded from monitoring programs. Ideally, these should be determined in a single analysis with polarity switching.
Furthermore, chemists analyzing pesticide residues are under increasing pressure to broaden the range of pesticides determined in a single analysis, to improve limits of detection, precision and quantitation, to increase confidence in the validity of residue data, to provide faster methods, and to reduce usage of hazardous solvents while maintaining or reducing costs. In order to meet these demanding requirements the scope, sensitivity, efficiency, and speed of multi-residue methods of analysis must be improved.
Given that there are many active ingredients used to control pests, it is often advantageous to extract and determine as many of them as possible during a single analysis. An extraction, with acetonitrile, followed by dispersive solid phase extraction (SPE) clean-up has been reported for the analysis of a wide range of pesticides in fruits and vegetables2 and fatty samples.3
A 10 g sample was weighed in a centrifuge tube. Acetonitrile (9.9 mL), acetic acid (0.1 mL), anhydrous MgSO4 (4 g), and sodium acetate (1.66 g) were added and the tube was shaken immediately. After centrifugation at 4,300 g for 5 min, an aliquot (1 mL) of the supernatant was transferred to a microcentrifuge vial containing 50 mg primary secondary amine (PSA) sorbent and 150 mg anhydrous MgSO4. The contents were vortex mixed for 30 s and centrifuged at 5,000 g for 1 min. The supernatant was analyzed by LC-MS/MS after dilution with water (1:9).
|
System: |
Waters ACQUITY UPLC |
|
Column: |
UPLC BEH C18, 2.1 x 50 mm, 1.7 μm |
|
Column temp.: |
40 °C |
|
Flow rate: |
0.6 mL/min |
|
Mobile phase A: |
Water + 0.1% acetic acid |
|
Mobile phase B: |
Methanol + 0.1% acetic acid |
|
Total run time: |
7 min |
|
Injection volume: |
20 μL |
|
Time: |
0 min |
90%A |
|
Time: |
4 min |
100%B |
|
Time: |
5 min |
100%B |
The Waters Quattro Premier XE Tandem Quadrupole Mass Spectrometer was used in positive and negative ion electrospray mode switching in 0.02 s. The ion source was operated at 120 °C with a capillary voltage of 1.0 kV. Nitrogen was employed for both the desolvation and cone gases at 800 (400 °C) and 50 L/hr, respectively. The mode of acquisition was multiple reaction monitoring (MRM) at an argon collision gas pressure of 4.0 x 10-3 mBar.
The Quattro Premier XE was tuned so that the precursor and product ions were resolved with a peak width at half height of less than 0.7 Da. The list of pesticide residues and the MRM transitions, along with the retention times, dwell times, cone voltages, and collision energies for the method are listed in Table 1. Pesticide residues listed in red type were acquired in negative ion mode. The dwell times were optimized so that ten to fifteen data points were acquired across each chromatographic peak.
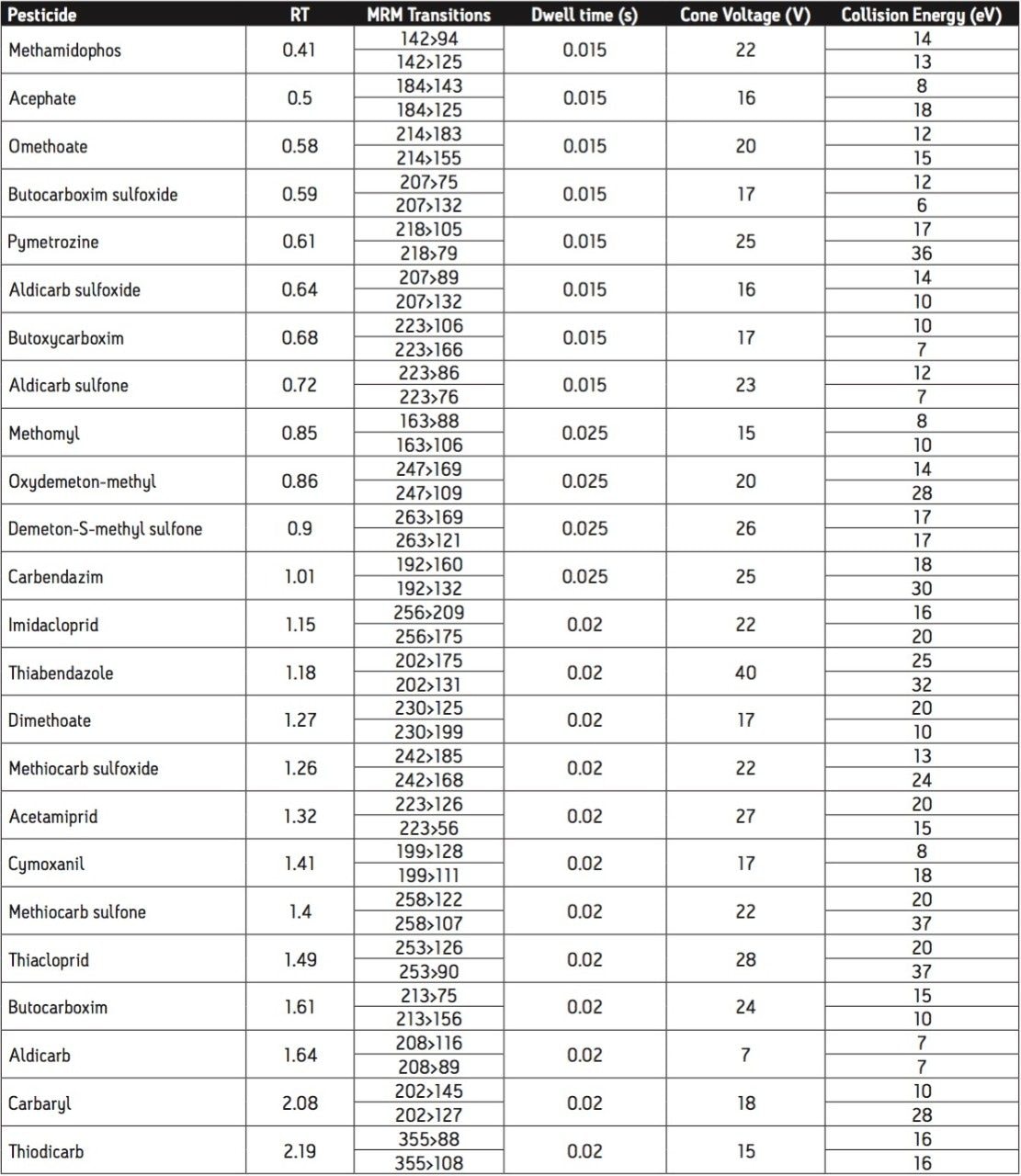
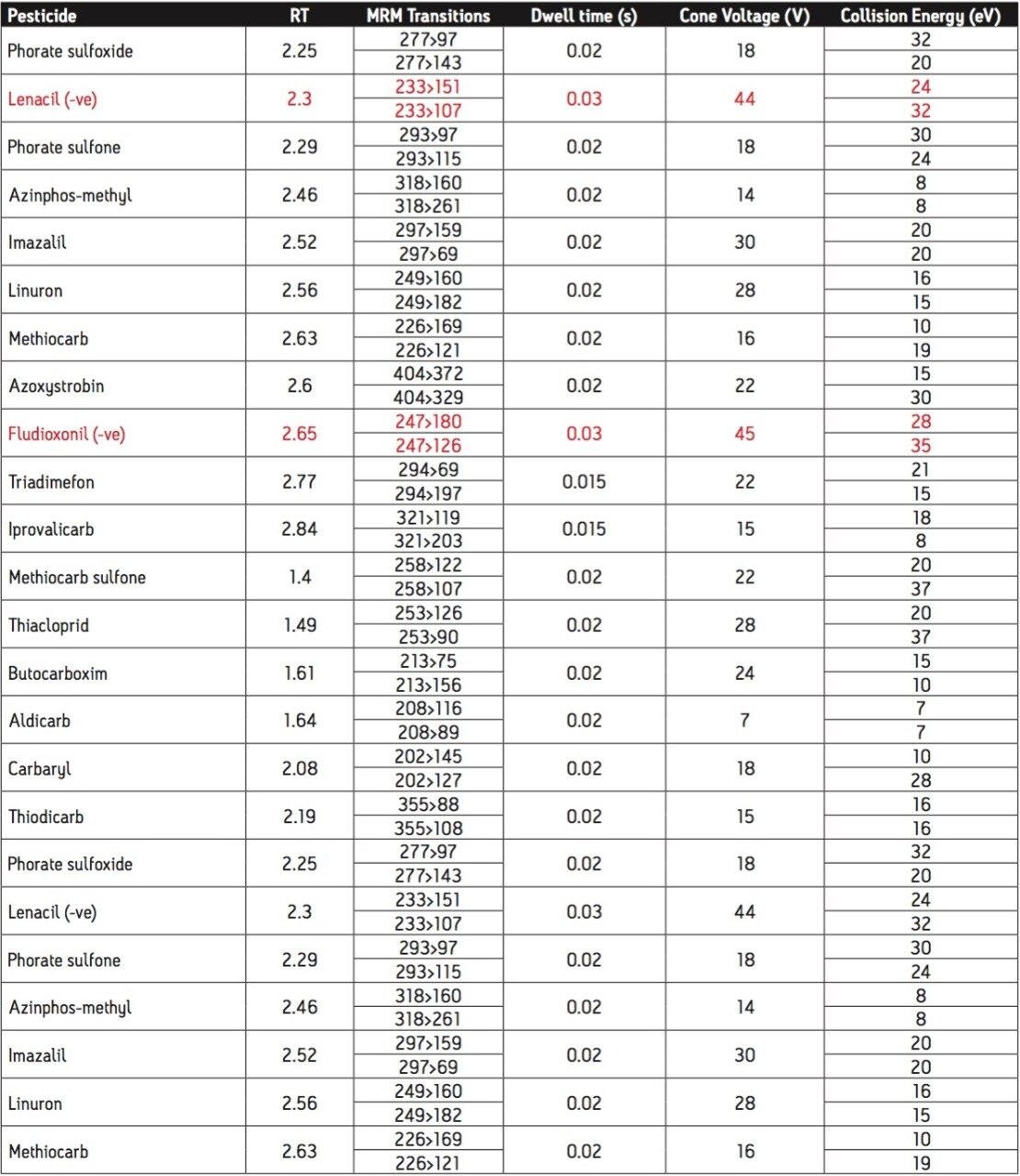

The data were acquired using Waters MassLynx Software and processed using TargetLynx Application Manager.
Two MRM transitions were acquired for each residue so that quantification and confirmation could be performed with a single injection assuming that the ion ratio between the two transitions is consistent for standards and samples. The confirmation criteria chosen were dependent on the relative abundance of the two transitions in accordance with Quality Control Procedures for Pesticide Residue Analysis.4
To test the extraction method described, five recovery experiments were performed in cereal-based baby food, spiked at 0.01 mg/kg. The mean recovery and relative standard deviation (% RSD) in parenthesis of each analyte are listed in Table 2.
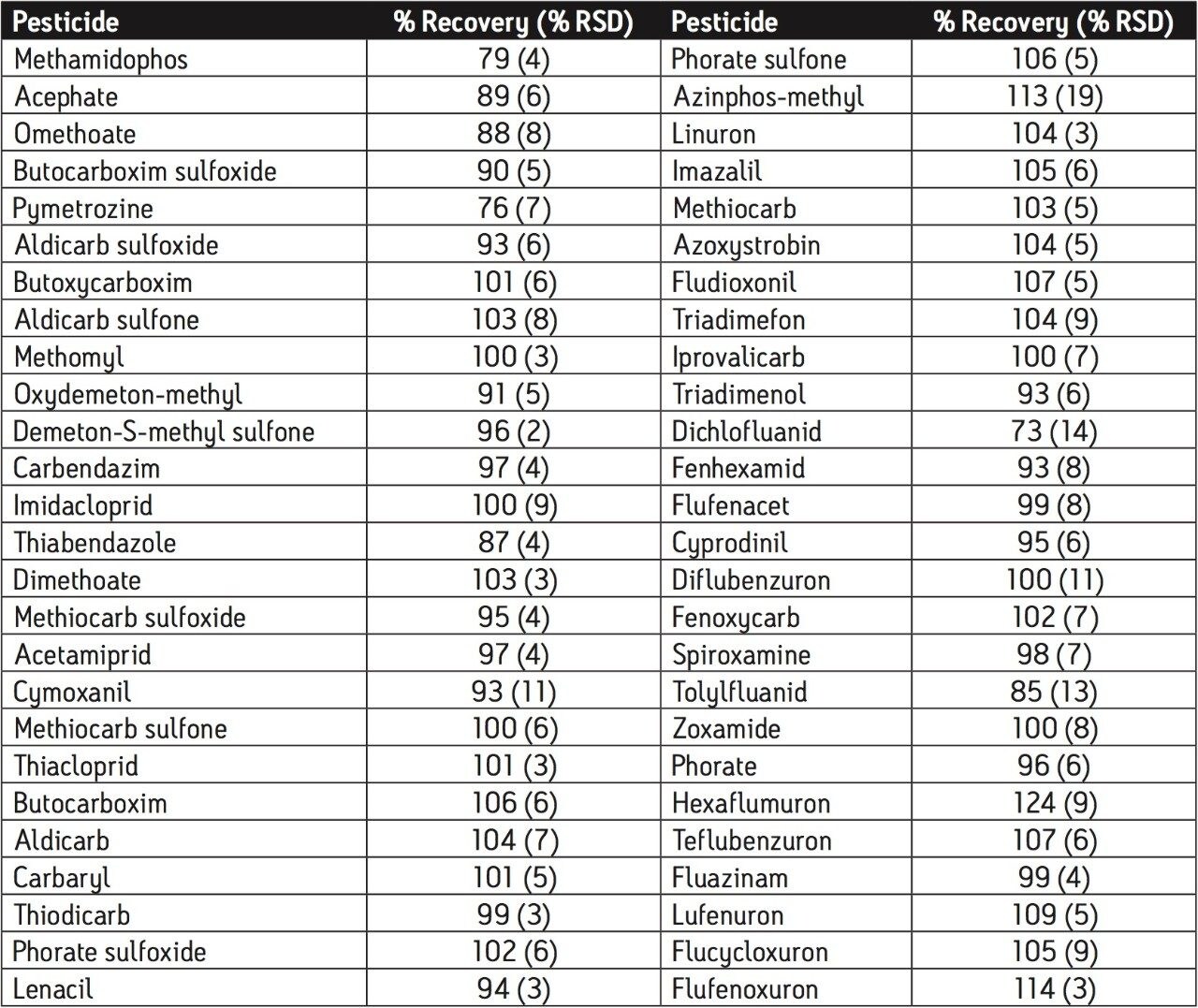
Good recoveries in the range 73 (dichlofluanid) - 124% (hexaflumuron) with % RSDs of less than 19% (azinphos-methyl) were obtained for all the pesticides spiked at the 0.01 μg/mL levels in cereal-based baby food.
The separation of the pesticides was optimized by changing the pH of the mobile phase. For multi-residue methods, the pH needs to accommodate different chemical properties, e.g. thiabendazole is a very basic compound and prefers low pH conditions. 5 mM ammonium acetate was originally used, however, this compromised the peak shape for thiabendazole. Acetonitrile with 0.1% acetic acid improved the peak shape for this compound, however, compounds such as tolylfluanid were retained on the column.
The final mobile phase contained methanol with 0.1% acetic acid, which gave a good peak shape for thiabendazole and allowed analysis of all analytes without compromising the response or the peak shape for the remaining pesticides. Dilution of the acetonitrile extracts with water also improved the peak shape and reduced any matrix effects in the extracts.
Using the UPLC method developed, the 52 pesticides of interest were eluted in less than four minutes (Figure 1) without a loss in resolution. An increase in the speed of the chromatographic separation by more than a factor of 10 was achieved using the described method compared to a typical HPLC separation time for approximately 50 pesticides of 50–60 minutes.
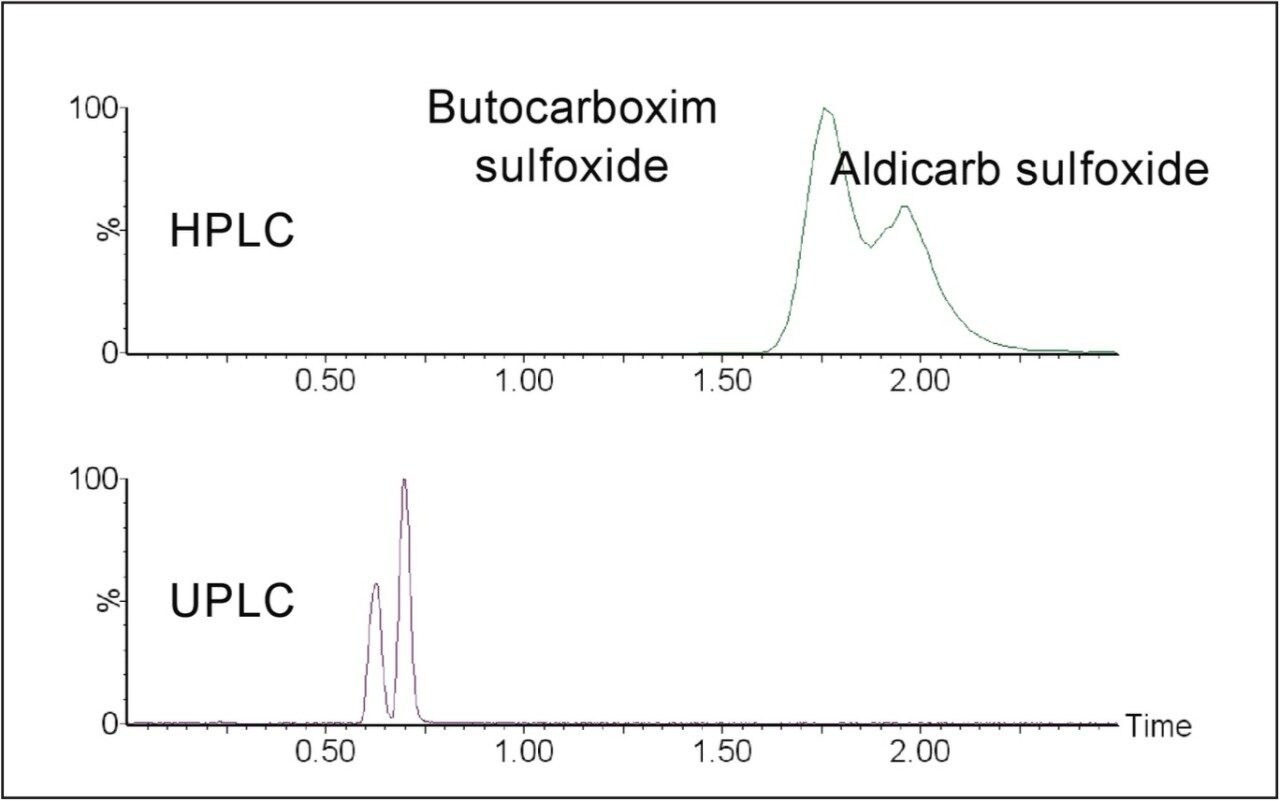
Butocarboxim sulfoxide and aldicarb sulfoxide are structural isomers that share one confirmation MRM transition (m/z 207>132) but differ in the quantification transition. However, one transition is not enough for confirmation. If they co-elute, chromatographic resolution is critical. Figure 2 illustrates the improved resolution achieved using UPLC (Rs = 1.3) compared to HPLC (Rs = 0.9) between the critical pair even though the gradient time is much shorter (20 min compared to 4 min). It is possible to obtain better resolution of the isomers using optimized conditions with HPLC but it then reduces the applicability of the method to such a broad range of compounds. The improved resolution offered by UPLC enables analysts to confidently confirm the identity of these two pesticides.
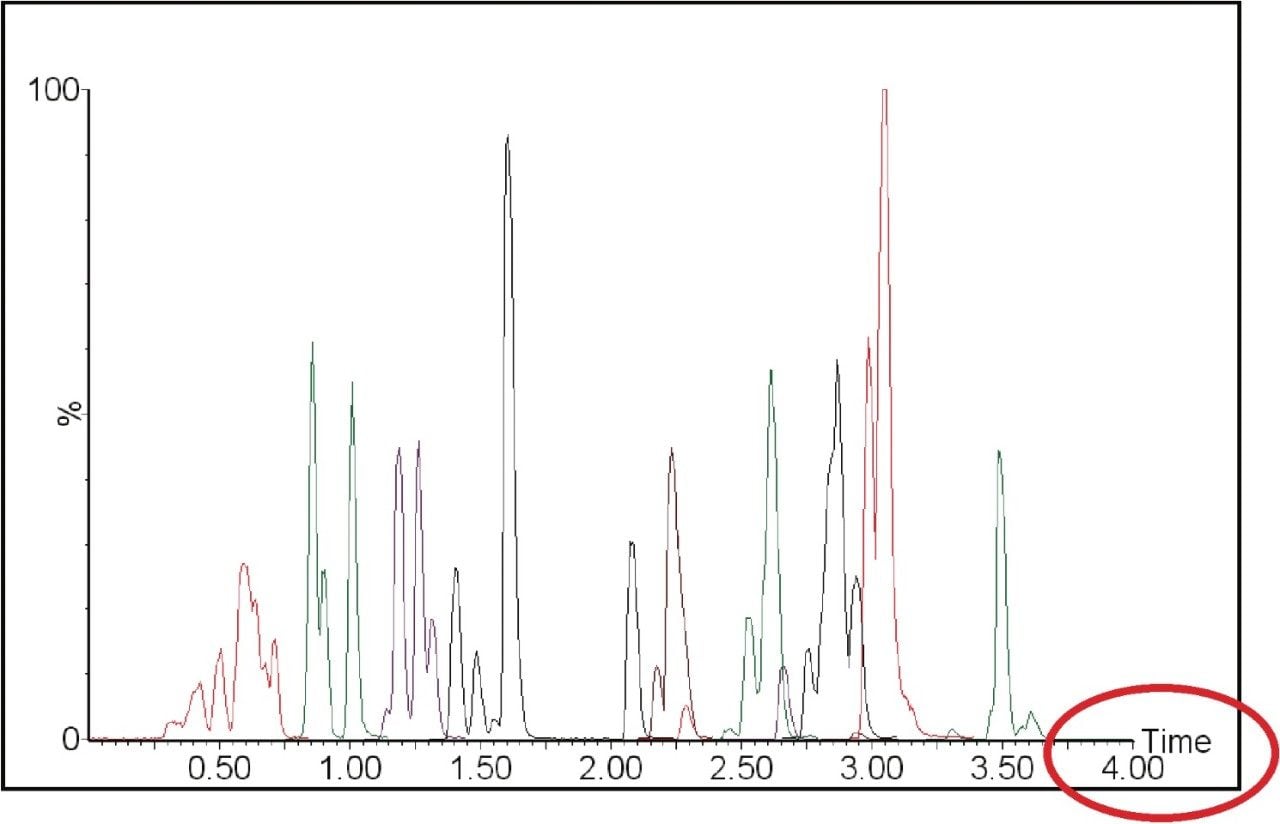
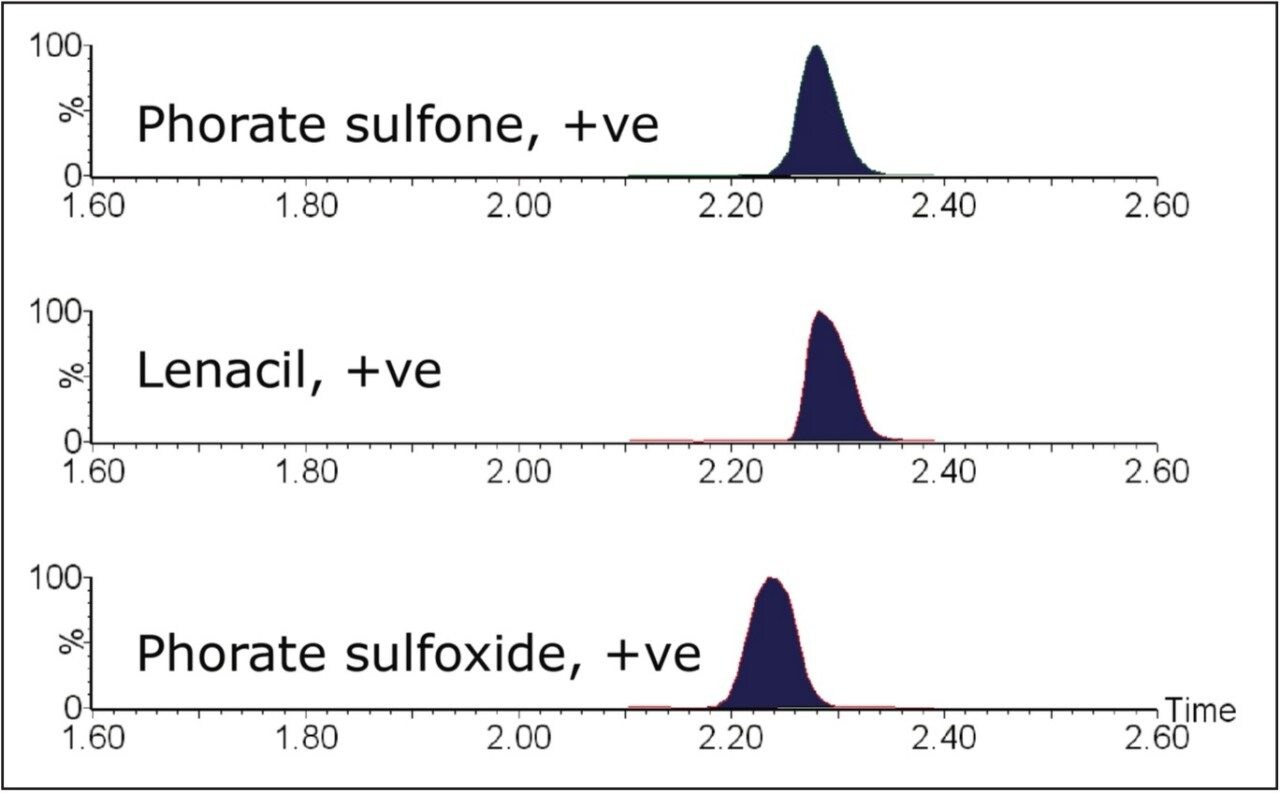
Using the UPLC method described, phorate sulfone and phorate sulfoxide (both positive ion compounds) co-elute with lenacil (a negative ion compound) in a time window of 9 s (Figure 3). To get 15 data points across each peak with two MRM transitions per compound, the overall cycle time for each transition, including dwell time, inter-scan delay, and inter-channel delay would need to be 100 ms. Older instruments require at least 200 ms just to switch, so the number of data points or the peak shape would have to be compromised to perform positive/negative switching in the same experiment.
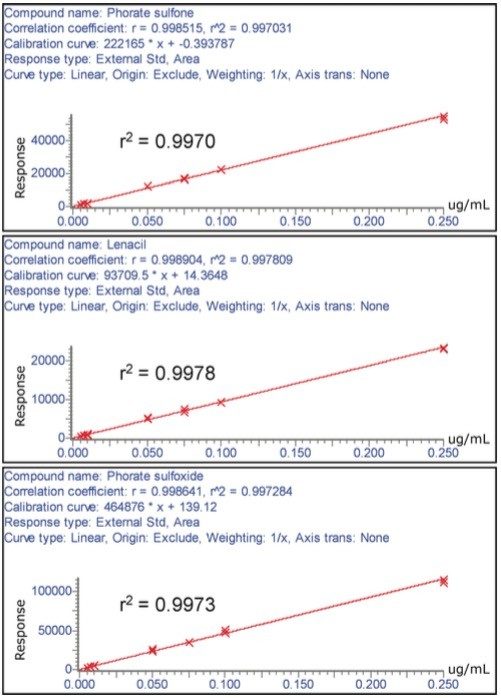
The ability to switch between positive and negative ion modes using a 20 ms interscan delay can be tested by observing the linearity of the calibration curve produced from a number of different concentration levels. The three matrix-matched curves for phorate sulfone, phorate sulfoxide and lenacil in cereal-based baby food between 0.005 and 0.250 μg/mL (equivalent to 0.005–0.250 mg/kg) are illustrated in Figure 4. Good correlation coefficients were obtained for all three compounds indicating that positive/negative switching can be achieved on the Quattro Premier XE using a 20 ms inter-scan delay.
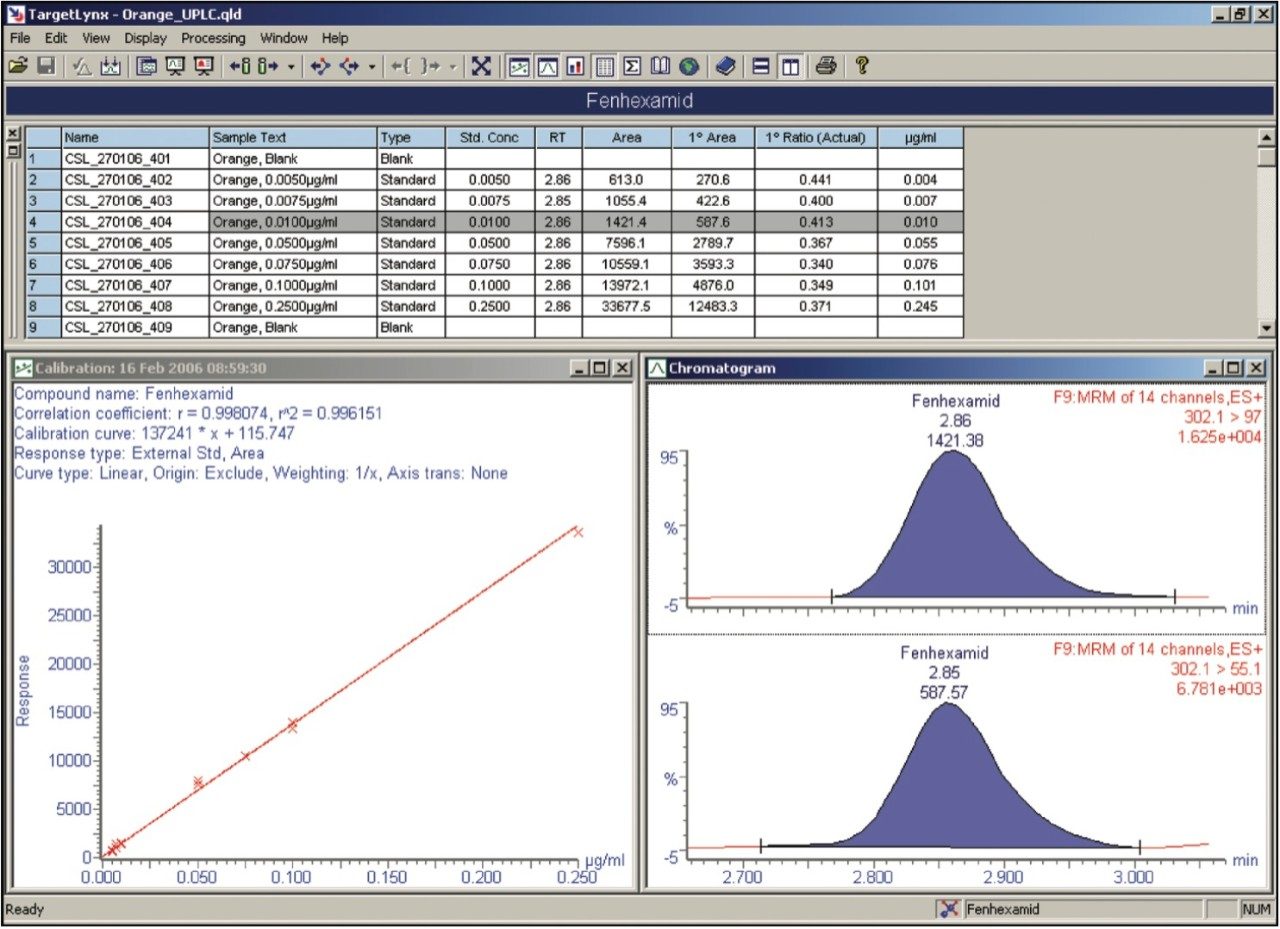
The TargetLynx Application Manager was used to provide automatic quantification and confirmation with two MRM transitions processed for each residue. The browser produced by TargetLynx for fenhexamid at a spiked concentration of 0.01 μg/mL in oranges is illustrated in Figure 5. Fifty-two residues could be screened and confirmed using this method in all three matrices to a concentration of 0.01 μg/mL.
The method was also applied to three potato samples containing suspected incurred pesticide residues. The samples were measured against the potato matrix-matched calibration standards. TargetLynx was used for automatic quantification and confirmation. The confirmation criteria chosen were dependent on the relative abundance of the two MRM transitions in accordance with Quality Control Procedures for Pesticide Residue Analysis.4 For illustration purposes the reporting level was chosen to be 0.005 mg/kg.
A Targetlynx browser showing two MRM transitions for incurred aldicarb sulfoxide is illustrated in Figure 6. In this example, the results indicate a confirmed concentration of 0.021 mg/kg. No interference can be observed on the confirmation transition (m/z 207>132) from butocarboxim sulfoxide, allowing unambiguous identification of the incurred residue.
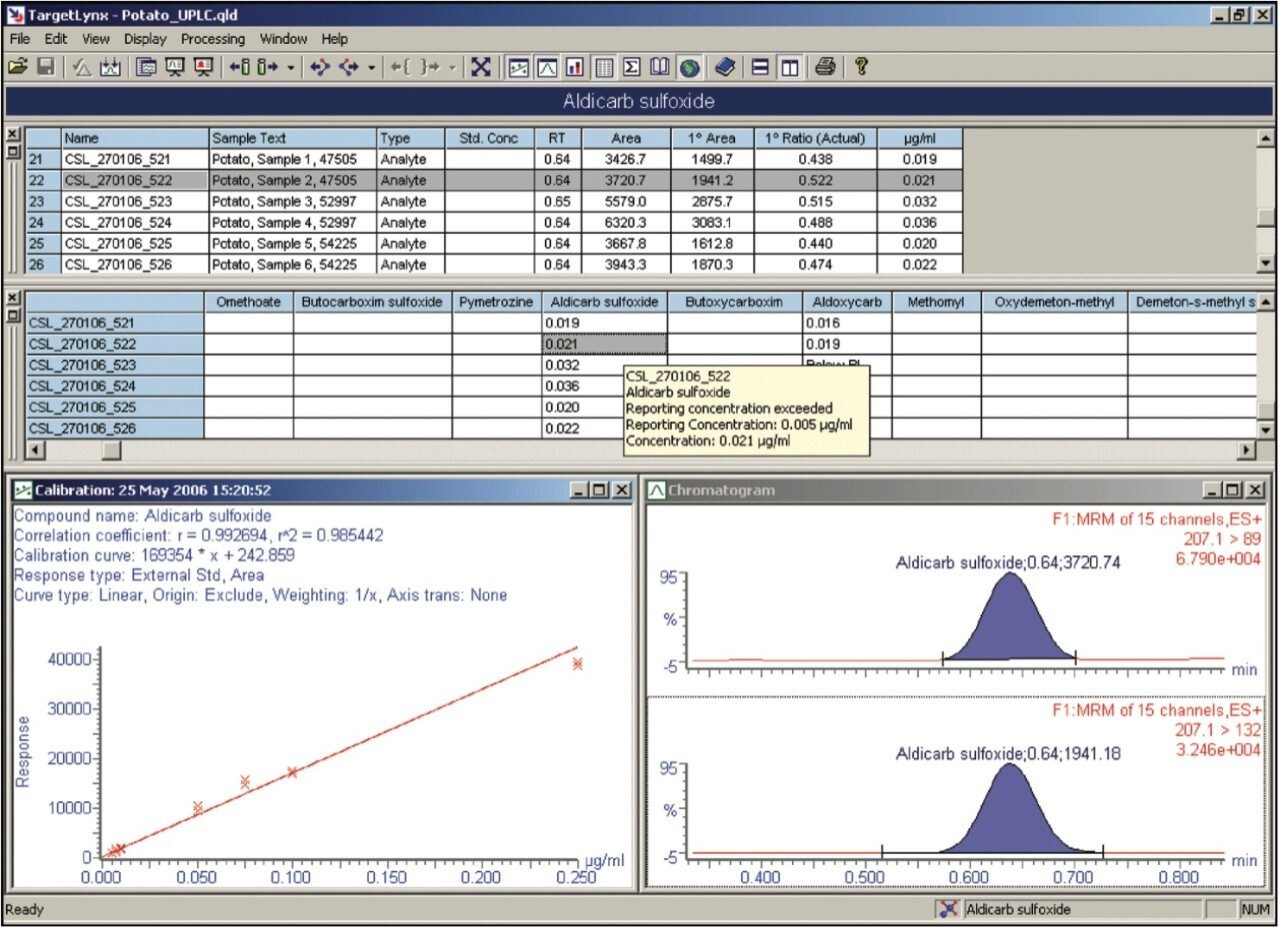
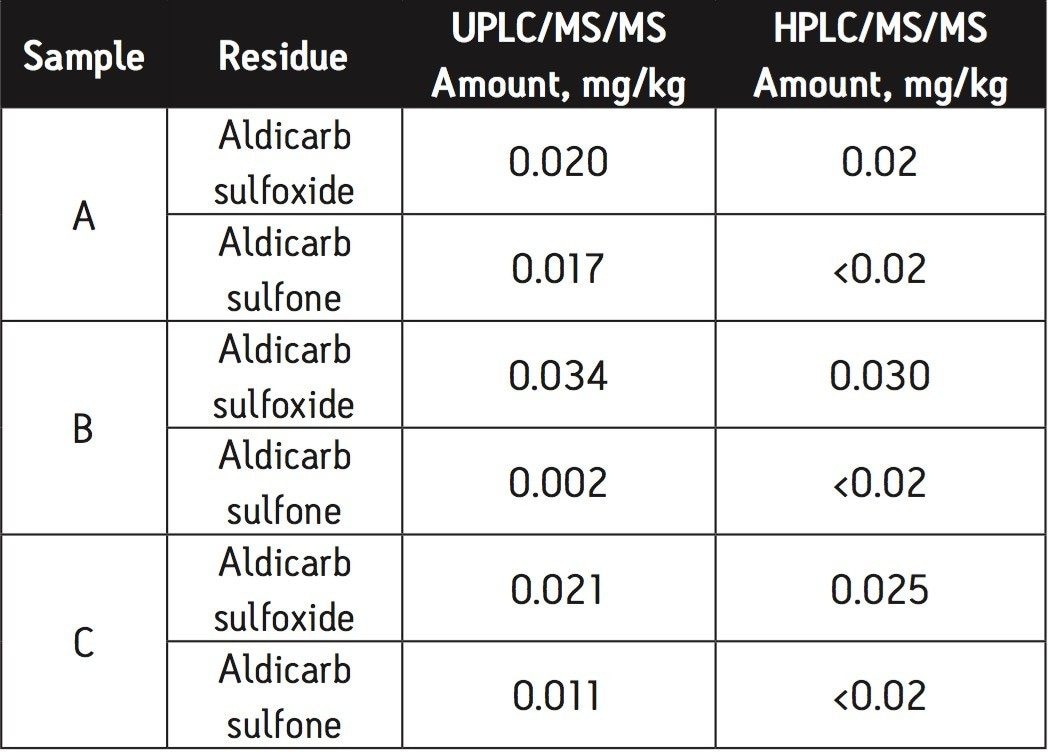
Table 3 lists the incurred residues detected in the three samples as an average of two injections. Aldicarb sulfoxide, and aldicarb sulfone (aldoxycarb) were detected with values above 0.002 mg/kg but below 0.035 mg/kg, not exceeding the UK, EU or Codex maximum residue limits of 0.500 mg/kg in potatoes. The correlation was good between results from the UPLC-MS/MS method and those from the established HPLC-MS/MS method used at CSL.
The tandem quadrupole instrument was capable of very fast polarity switching, allowing the analysis of positive and negative compounds in a single run.
The UPLC method showed significant advantages over traditional HPLC methods including a ten-fold increase in the speed of the separation without the loss of resolution, as shown by the separation of the structural isomers butocarboxim sulfoxide and aldicarb sulfoxide.
A fast and simple UPLC-MS/MS method involving polarity switching has been developed for the determination of 52 pesticides. Of these, 21 pesticides and 7 metabolites are included in the 2005-2007 EU residue monitoring program. The remaining 23 pesticides and metabolites were included because they occur as residues, work in negative ion electrospray mode, or were used to demonstrate chromatographic resolution.
The described method gave satisfactory recoveries for the majority of pesticides in potatoes, oranges, and cereal-based baby foods.
720001995, August 2007