This is an Application Brief and does not contain a detailed Experimental section.
This application brief demonstrates the capabilities of the integrated UPLC-MS analysis of an intact monoclonal antibody with a comprehensive platform, the Biopharmaceutical Platform Solution with UNIFI, for accurate mass measurement, data processing, and reporting with UNIFI Scientific Information System.
This UNIFI-based platform addresses previous limitations with a comprehensively integrated platform for data acquisition by chromatography and mass spectrometry, with automated reporting.
The growing biotherapeutic pipeline means that the efficient characterization of monoclonal antibodies (mAb) is of growing importance, both to regulatory authorities and to pharmaceutical companies. Being able to perform acquisition and processing within the same platform, complete with an audit trail, is an important goal for regulated environments.
Accurately identifying post-translational modifications such as protein glycosylation is required as part of guidelines as they play several key roles in biological systems. Fast and accurate analysis of the glycoproteins is required in order to ensure the safety and efficacy of the biotherapeutic.
The ACQUITY UPLC H-Class Bio’s high-resolution bioseparations combined with high mass accuracy mass spectrometry detection with the Xevo G2 Tof provides routine UPLC-MS applications for biopharmaceutical laboratories.
There is a large set of data generated during each mAb analysis requiring interpretation of a variety of glycosylated forms and comprehensive characterization of the final product. This step sets productivity limits to otherwise high-throughput procedures and hinders automation of the process.
The UNIFI-based platform addresses these limitations with a comprehensively integrated platform for data acquisition by chromatography and mass spectrometry, with automated reporting.
To solve the problem of time-consuming data analysis and facilitate data processing of therapeutic mAb, the Biopharmaceutical Platform Solution with UNIFI was configured for the study of intact proteins. This represents a holistic approach of UPLC-MS data acquisition followed by automatic processing and annotation of the data in a high-throughput manner, which are further exported for data management.
UPLC-MS analysis of the mAb Trastuzumab was performed automatically. Aqueous solutions of 0.1% FA and 0.1% FA solution in acetonitrile were used as eluents A and B, respectively. Column temperature set to 80 °C is critical for successful chromatographic separation. The system included an ACQUITY UPLC H-Class Bio, an ACQUITY UPLC Protein BEH C4 Column, and a Xevo G2 Tof. The UNIFI Scientific Information System for acquisition, data processing, and reporting completes this comprehensive Biopharmaceutical Platform Solution.
The intact protein analysis report demonstrates the report objects, which can be entirely configured by the user: TIC summarized chromatogram; raw, deconvoluted, and centroid mass spectra; and tabulated summary of the interpreted LC-MS data (Figure 1). This detailed view shows an example of a deconvoluted spectrum within a specified mass range and parameter settings defined in the method. Deconvolution reveals several core glycosylated species which match the number of glucose residues and level of fucosylation. Another report object is a table with mass measurement of the intact mAb and accurately assigned mAb glycan variants (Figure 2). Mass errors were reported for each Trastuzumab MS peak with a corresponding retention time entry from the TIC chromatogram.
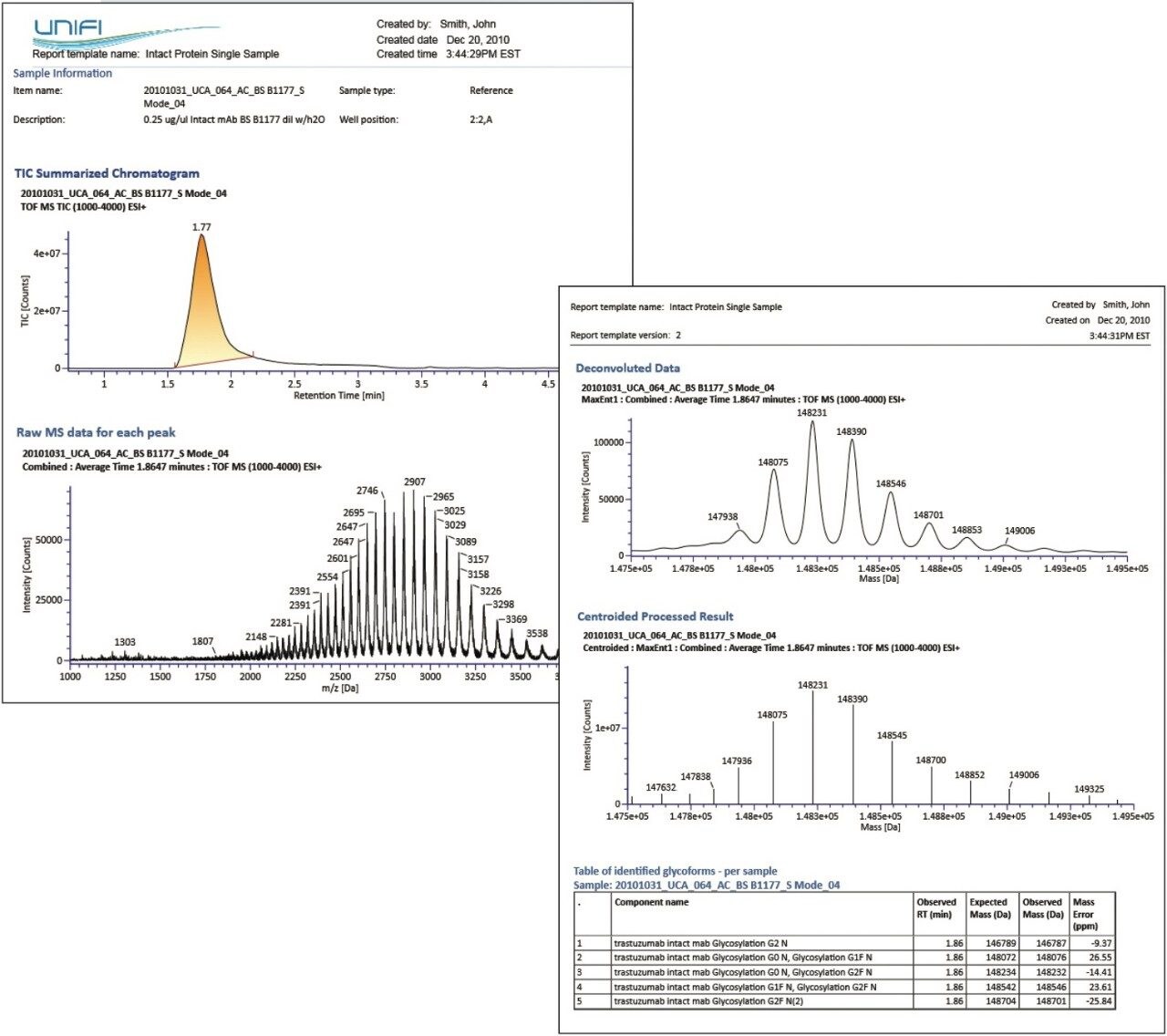
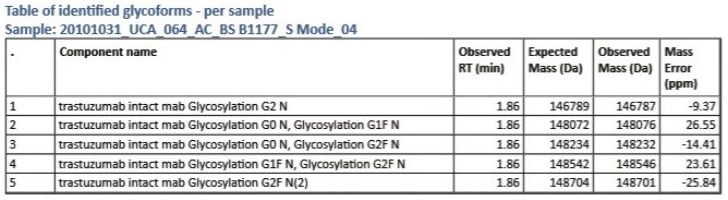
Such an integrated LC-MS approach provides the user flexibility to work with both raw and processed data followed by quick and efficient data management.
The capabilities of the Biopharmaceutical Platform Solution with UNIFI have been successfully demonstrated with the example of an intact biotherapeutic mAb.
Modern instrumentation and evolving analytical techniques extend the limits of the biopharmaceutical industry and consequently impose strict control of manufacturing processes.
Highly efficient and cost-effective integrated UPLC-MS approaches with the UNIFI Scientific Information System for data processing and reporting satisfies regulatory requirements and facilitates intact protein characterization. This technology covers the range from detailed structural protein characterization to sophisticated data management with UPLC-MS platforms.
720003843, January 2016