
For forensic toxicology use only.
This is an Application Brief and does not contain a detailed Experimental section.
With the emerging interest in cannabinoids, there is an increased demand for analytical methods capable of separating them to meet the strict regulatory requirement.1 The lack of consensus analytical methods for cannabinoids testing allowed scientists to explore advanced technologies. Herein, this technical brief provides a method with good separation of 7 cannabinoids based on UltraPerformance Convergence Chromatography technology, in under 10 min. Combined with the use of Viridis BEH 2-EP Column and CO2 as mobile phase with less solvent waste produced, this developed method provides excellent reproducibility and a greener alternative, making this a valuable complementary analysis to reversed-phase liquid chromatography.
Cannabis, also known as marijuana, has seen a steady increase in legalization for use for both health and recreational purposes, over the recent decade. Cannabinoids which are chemicals found in cannabis are thus extensively studied by scientists around the world. With this emerging interest in cannabinoids, there has been a tremendous demand for analytical techniques that can separate and quantify the components of interest.
Reversed-phase liquid chromatography (RPLC)2,3 has predominantly been used for the analysis of various cannabinoids and, in recent years, there is also an increased trend to use liquid chromatography with mass spectrometry (LC-MS)4 to analyse these substances as it offers higher selectivity for co-eluting compounds versus UV detection alone.
Beyond the use of liquid chromatography, UltraPerformance Convergence Chromatography (UPC2) was explored as an orthogonal analysis for cannabinoids. Together with the use of sub-2-µm particle columns and various detectors, the use of UPC2 enables rapid detection and separation. UPC2 is a holistically designed chromatographic technology that uses compressed CO2 as the primary mobile phase to leverage the chromatographic principles and selectivity of normal-phase LC, while providing the ease-of-use and method development simplicity of reversed-phase LC. As such, the retention order of elution is typically the inverse of the reversed-phase LC and this orthogonality is valuable for confirming analyte identity in complex matrices.
In this application brief, we describe the development of an analytical method using ACQUITY UPC2 and PDA for the separation of 7 cannabinoids, in less than 10 min.
|
Instrument: |
ACQUITY UPC2 with ACQUITY PDA Detector |
|
Software: |
Empower 3 |
|
Column: |
Viridis BEH 2-EP 130 Å, 100 x 3.0, 1.7 µm |
|
Column temp.: |
50 °C |
|
Sample temp.: |
5 °C |
|
ABPR: |
2000 psi |
|
PDA channel: |
228 nm |
|
Mobile phase: |
Gradient Elution with A: Carbon Dioxide B: 1:1 Methanol:Ethanol |
|
Run time: |
10 mins |
|
Injection volume: |
1 µL |
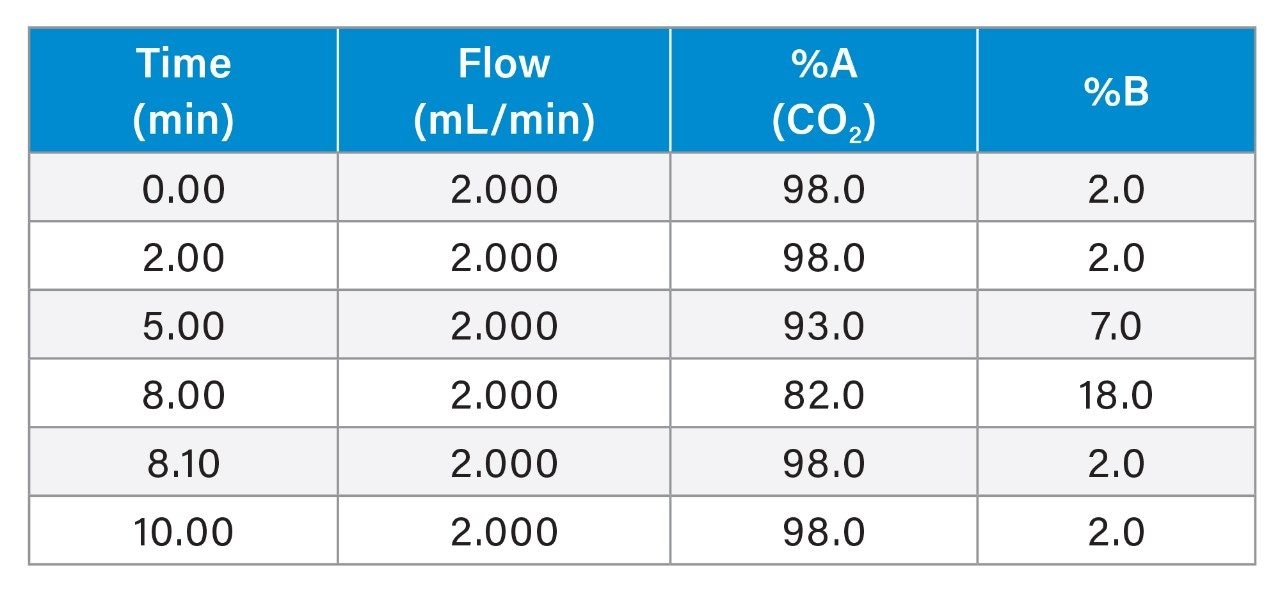
A mixture containing 7 Cannabinoids standards Δ9-THC, Δ8-THC, CBC, CBD, CBN, CBG, and THCA were dissolved in methanol (Figure 1) and analyzed in replicate (n=6). Separation of these cannabinoids was achieved using an ACQUITY UPC2 System coupled with an ACQUITY UPC2 PDA Detector in 10 min, using a gradient elution of carbon dioxide (CO2) and a mixture of methanol and ethanol.
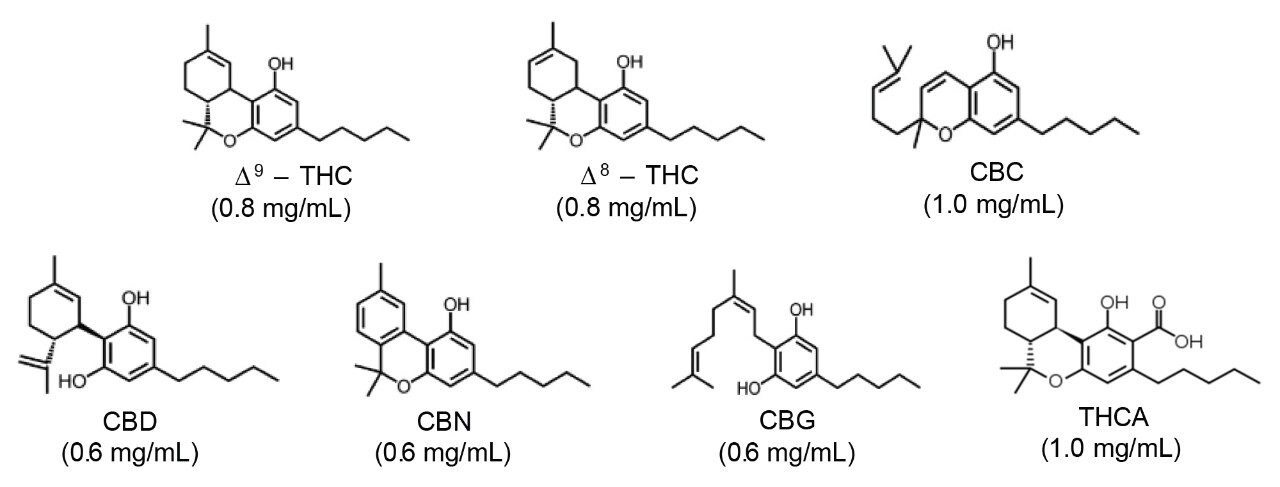
A Viridis BEH 2-EP Column (P/N 186006581) was utilized for this separation. The column stationary phase consisting of ethylene bridged hybrid (BEH) with 2-ethylpyridine (2-EP) provides chromatographic characteristic well suited to the separation and retention of the cannabinoids, under these UPC2 conditions (Figure 2).
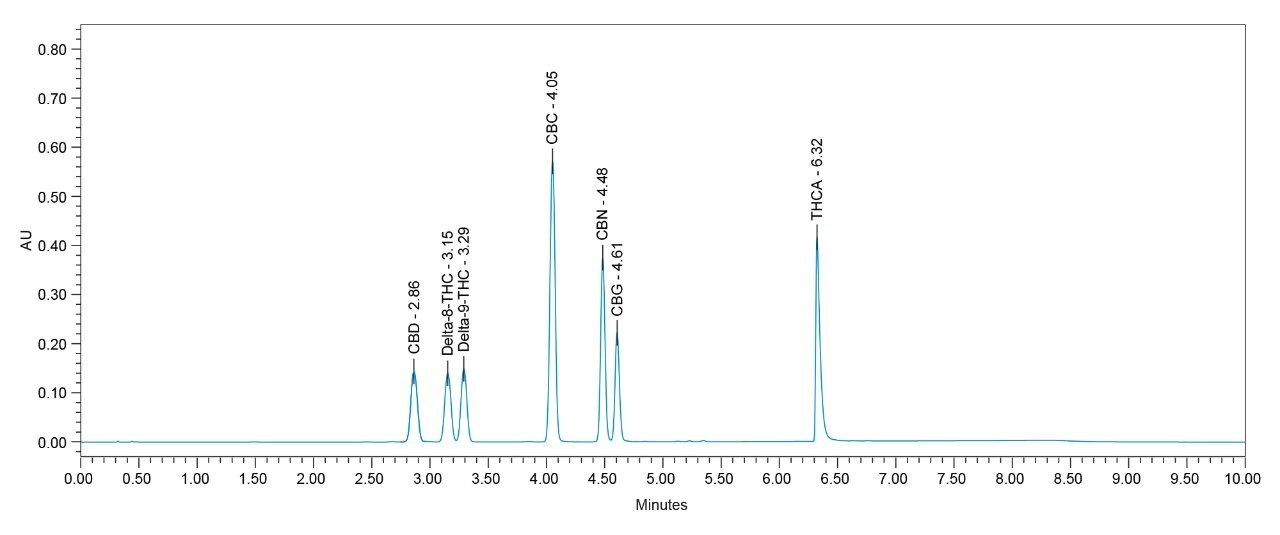
The proposed methodology provides good chromatographic separation and retention of the 7 cannabinoids in a single method. The peaks of interest are well separated with peak resolution greater than 1.40 for the closely eluting achiral Δ9-THC and Δ8-THC and peak resolution greater than 1.65 for the remaining cannabinoids (Figure 3).
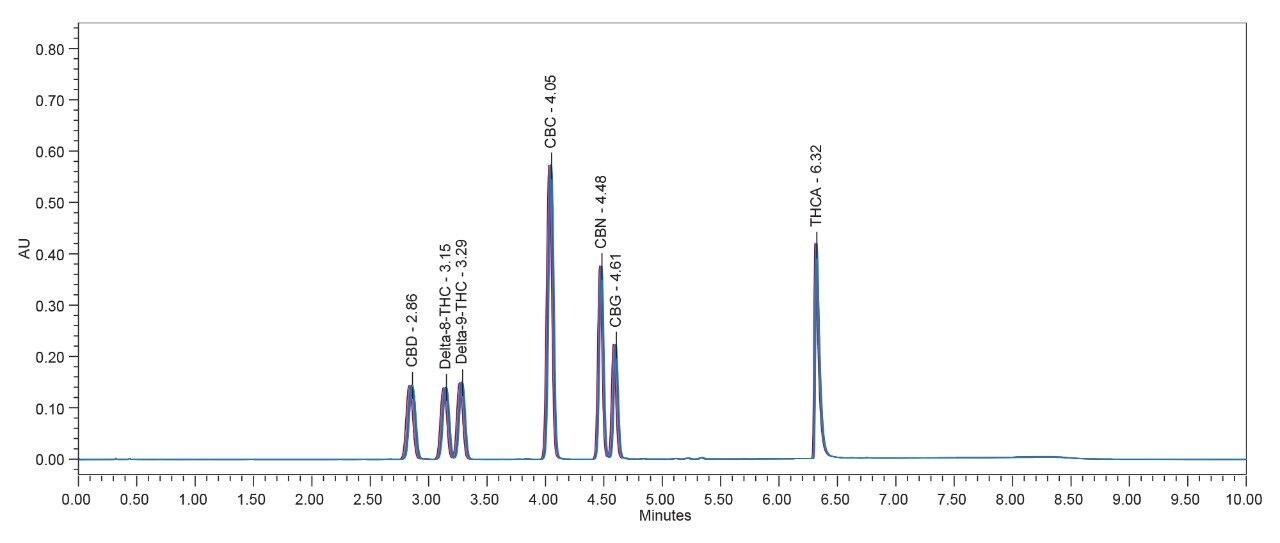
Excellent area reproducibility (RSD 0.3%-0.7%) for all the cannabinoids analytes of the 6 injections demonstrated the superior stability and high precision (1 µL injection volume) of the ACQUITY UPC2-PDA System in analyzing the cannabinoids of interest. This method also gives retention time stability of ±0.05 min.
An ACQUITY UPC2-PDA method for the detection of cannabinoids Δ9-THC, Δ8-THC, CBC, CBD, CBN, CBG, and THCA has been developed. It demonstrates excellent reproducibility and good separation of the 7 cannabinoids. Combining the retention capability of the Viridis Column for cannabinoids together with the resolving power of the ACQUITY UPC2-PDA System, this configuration provides an efficient and rapid separation method that is orthogonal to the conventional RPLC-PDA analysis of cannabinoids. Thus, providing a strategy to manage substances that may co-elute using conventional RPLC separation.
720007230, April 2021