
This is an Application Brief and does not contain a detailed Experimental section.
Ion-Exchange Chromatography (IEX) for proteins has historically been incompatible with mass spectrometry (MS) due to the use of high concentrations of non-volatile salt. IEX-MS has become a reality through the replacement of traditional salts with MS-friendly volatile salts. As such, new considerations should be addressed with the change of mobile phases. This brief seeks to help give practical guidance for IEX-MS from an instrumental perspective.
Ion-exchange chromatography is a separation technique that is gaining greater traction in the realm of mass spectrometry. Unique challenges present themselves due to the use of salty aqueous solutions. Beyond previous considerations for both the mobile phase and sample preparation,1 it is important to consider both the liquid chromatography (LC) and mass spectrometer (MS) holistically.
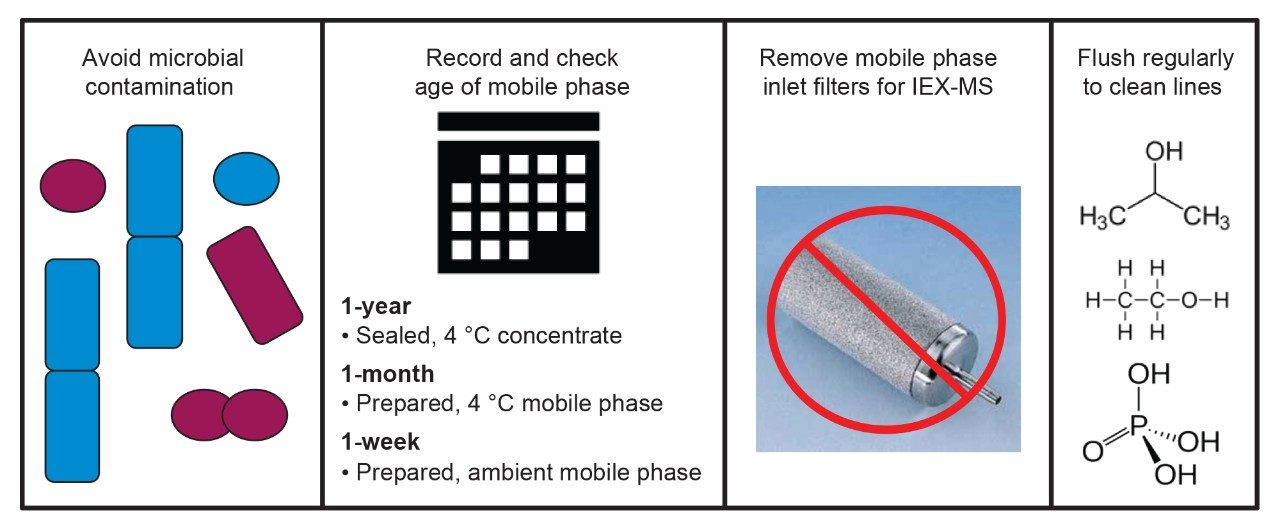
The use of aqueous mobile phases introduces the potential for microbial growth and contamination, which can have detrimental effects on chromatography and instrument lifetime. Good lab hygiene and maintenance must be utilized to avoid this persistent and pernicious problem. Those familiar with other native aqueous separations, such as size-exclusion chromatography (SEC), should be well versed in the need for avoiding contamination. Simple regimented cleaning routines can help ensure optimal performance of IEX-MS. Furthermore, dedicated parts can also make a significant improvement if a single instrument is used for a variety of chromatographic modes.
Native MS-friendly ion-exchange buffers are generally ammonium-based solutions, with ammonium acetate-based compositions, such as Waters IonHance CX-MS Buffers (p/n: 186009280, 186009281). These are generally preferred for a number of reasons.2 Consideration needs to be given to the fact they these solutions have favorable enough conditions for microbial growth to occur. The easiest approach is to think of prepared aqueous solutions as having a timer that is activated when prepared. Typically, it is suggested that aqueous mobile phases (e.g., diluted and prepared IonHance CX-MS Buffers) not be left on a system at room temperature for longer than one week due to the danger of microbial contamination. When prepared, the bacteriostatic acetonitrile concentration of the packaged concentrates is diluted to a point where microbial life can proliferate. As such, once the buffers are prepared, it is suggested that the solutions be kept refrigerated (i.e., 4 °C) when not installed on an LC system. Buffers that are prepared and refrigerated can be kept for approximately one month.
Beyond the mobile phases themselves, it is possible for microbial life to contaminate a system and column. Once a column is contaminated, the column should be discarded. To prevent such an occurrence, it is recommended that the column is stored as suggested by the manufacturer, and that the LC lines used be purged with a strong organic-water solution (e.g., 70% isopropanol 30% 18.2 MΩ water) for any period of inactivity, such as over weekends. Regular (e.g., weekly) flushing with organic helps prevent microbial colonies from establishing footholds within the LC system. If a system is contaminated, the use of strong organics and/or acids (e.g., 20% phosphoric acid) may prove useful and can help ameliorate the situation.
Mobile phase inlet filters are highly porous structures that provide an easy haven for microbial life and growth. It is suggested that the inlet filters be removed from the terminus of the LC line for native LC-MS work. The end of the line can simply be positioned into the mobile phase reservoirs while extra care is giving to using only high quality solvents and mobile phase reagents.
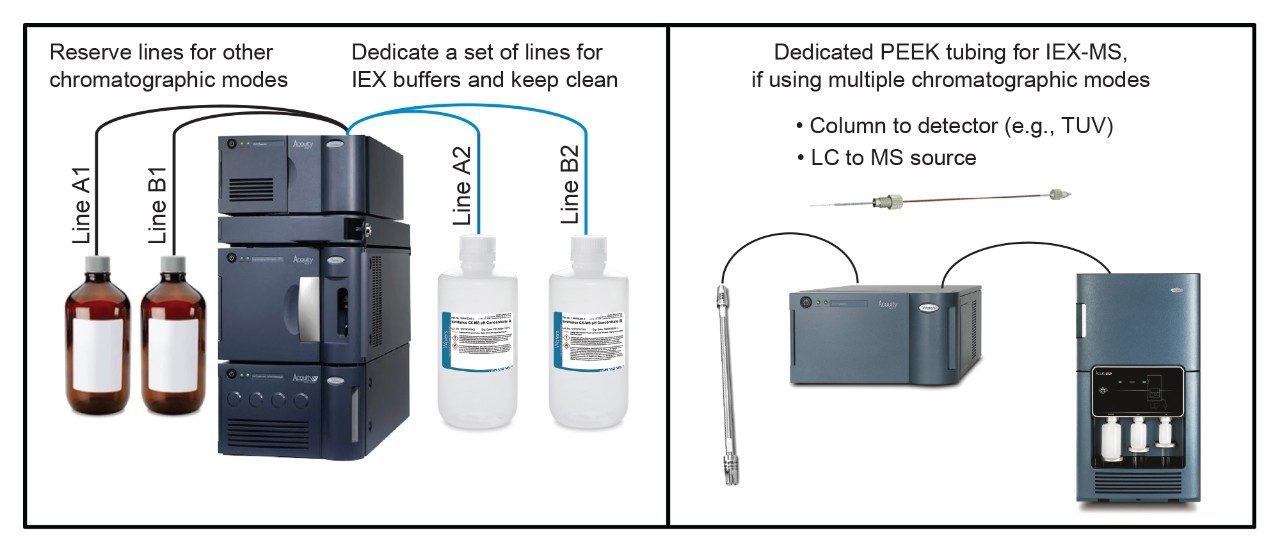
If using an LC-MS system for multiple chromatographic separations, please note that trifluoroacetic acid (TFA) appears to have a detrimental effect on the performance of IEX-MS. Flushing TFA out of a system can take a considerable amount of time. It is therefore recommended to dedicate LC lines for reversed phase and IEX (e.g., A1/B1 for reversed phase with TFA and A2/B2 for IEX-MS), if possible. Furthermore, the use of dedicated PEEK capillaries for connecting the column to the TUV and from the TUV to the MS can make the transition between reversed phase and IEX easier and with improved sensitivity for IEX-MS. The allocation of dedicated PEEK tubing allows for a quick and seamless transition between separation modes without the need for time intensive cleanings.
As previously shown, IEX-MS is highly sensitive to contamination. To help ensure peak performance, it is suggested that the LC be cleaned regularly, especially if a LC-MS system is used for both IEX and alternative separation techniques. We recommend monthly flushing with a 25/25/25/25 percent solution of water/acetonitrile/isopropanol/methanol if using the system solely for IEX-MS and at any point that the system is switched between chromatographic modes.
In IEX-MS with a 2.1 mm I.D. column, the operating LC flow rate can be varied between 50–250 µL/min. The use of lower flow rates in ESI-MS has proven advantages for the detection of biomolecules. The use of higher flow rates may also have a negative impact on chromatographic resolution. When changing flow rate, care should be given to achieve separations with reasonable gradient steepness values in terms of ΔpH/ΔCV (column volume).
Though the use of volatile aqueous buffers allows for MS detection following native LC separation, the technique is less sensitive than methods using acid or base-modified water/organic mobile phases. Therefore, optimal MS tuning parameters and careful system maintenance are necessary for success. For more detailed IEX-MS method guidance and tuning, please refer to the application notes in the References section.1-4
For maintenance, a monthly inspection of the MS source enclosure is a reliable measure to ensure no salt deposits are forming. For Tof and QTof instruments, a quick cleaning protocol is to simply wipe the surface of the source enclosure with a lint free tissue containing MS-grade methanol. If the cone shows salt deposit, it is best practice to clean the cone following the operator’s overview and maintenance guide for the instrument in use. For the BioAccord System, the replacement of the aperture disc is required when the system health displays that there is a blockage or that sensitivity has been reduced.
A dedicated ESI probe capillary would also make the transition from reversed phase to IEX smoother. This is akin to the dedicating post-column PEEK tubing. The use of a dedicated ESI probe allows for a simple swap without the need for extensive flushing to ensure optimal signal for IEX-MS.
A once yearly planned maintenance (PM) visit is sufficient for an instrument, provided that care is taken to keep the system clean of biological materials or salt build up. From an everyday perspective, clean solvents (e.g., IonHance CX-MS Buffers), mobile phase bottles (i.e., MS quality thermoplastics), and ensuring appropriate sample loading to the column are more important.
Lastly, it is recommended to establish a system suitability test (SST) sample (e.g., Humanized mAb Mass Check Standard, p/n: 186009125) for which expected signal intensity, signal-to-noise, and data quality can be monitored with each run. If a decrease in data quality is observed, the system may require any or all the maintenance steps outlined above.
It has been observed that injection of a smaller volume of a more concentrated sample (e.g., 1 μL of 10 mg/mL) tends to lead to better data compared to a larger volume of a more dilute sample (e.g., 10 μL of 1 mg/mL). As such, it is recommended to use a more concentrated sample as opposed to diluting a sample. Furthermore, it is preferable to prepare the sample in the operating mobile phase. For the preparation of the mobile phases, 18.2 MΩ water, such as Milli-Q water, is the preferred diluent for the mobile phase concentrates. The use of glass bottled water may be a source of metal ions. A general avoidance for glass mobile phase bottles, cylinders, and sample vials can help minimize salt/metal adducts and ultimately achieve better results.
Native LC-MS with aqueous mobile phases suffers detrimental effects on data quality and instrument health when microbial contamination is introduced. The good hygiene protocols and suggested maintenance in this document will improve data quality and reduce the down time of instruments due to poor sensitivity or loss of chromatographic resolution as a result of contamination or salt deposits. If you are still struggling with poor data quality or instrument contamination, please contact Waters Technical Support services via iRequest.
720007144, February 2021