For research use only. Not for use in diagnostic procedures.
The direction of glucose in the same cell line can change dramatically depending on whether they are grown as 2D monolayers or 3D spheroids. In this application note, we show the results of a small case study where Symphony was interfaced to PollyPhi for the processing and analysis of isotope labeling metabolic relative flux data of 2D and 3D non-small cell lung carcinoma cells lines treated with 13C-glucose.
We illustrate the use of Symphony Software and Polly Software for contrasting the flow of glucose within mammalian cells grown in vitro in two and three dimensions, monolayers and spheroids, respectively. The more commonly used two-dimensional cell cultures are believed to be inadequate to recreate the in vivo physiologically relevant microenvironment.
Mammalian cell lines are commonly used to study human disease and treatment using (2D)/monolayer cell cultures. However, this geometry of multicellular cell cultures is believed to be inadequate to recreate the biological microenvironment of naturally occurring cells, tumor cells in particular.1,2 Three-dimensional (3D)/spheroid cell cultures are considered to be a more viable in vitro alternative since they better mimic the in vivo cellular growth environment. The potentially different outcomes from experiments using 2D and 3D culture systems may have a significant impact on the relevance of experimental findings. For example, the effectiveness of a drug treatment studied in (2D)/monolayers does not necessarily predict equivalent effectiveness in vivo. In this application note, we show the results of a small case study where Symphony was interfaced to PollyPhi for the processing and analysis of isotope labeling metabolic relative flux data of 2D and 3D non-small cell lung carcinoma cells lines treated with 13C-glucose.
H1299 cell lines were grown and extracted as previously described.3 Briefly, cell lines were grown as monolayer in complete Dulbecco’s Modified Eagle’s Medium to generate spheroids, cells were grown in Falcon Bacteriological Petri Dishes coated with 2% pHEMA dissolved in 100% ethanol. Monolayer cells were dissociated using 0.25% Trypsin-EDTA, whereas spheroid cultures were dissociated using StemPro Accutase. Cell extracts from unlabeled cells or from cells labeled with [U-13C] glucose were pelleted and re-suspended in water and lysed by heat shock treatment. Subsequently, chilled MeOH was added followed by the addition of CHCl3. The samples were centrifuged and the resulting phases separated, followed by the addition of chilled acetonitrile and overnight incubation. The samples were centrifuged again and the supernatant collected and dried. Next, the samples were re-suspended and injected onto the LC-MS system. A graphical overview of the experimental design is shown in Figure 1.

|
LC system: |
ACQUITY UPLC I-Class |
|
Column: |
BEH C18, 1.7 μm, 2.1 mm × 50 mm (p/n 186003685) |
|
Column temp.: |
40 °C |
|
Sample temp.: |
4 °C |
|
Injection volume: |
2.0 μL |
|
Flow rate: |
0.4 mL/min |
|
Mobile phase A: |
0.1% Formic acid in water |
|
Mobile phase B: |
0.1% Formic acid in acetonitrile |
|
Mobile phase D: |
0.1% Formic acid in IPA/acetonitrile (90/10, v/v) |
|
Time |
%B |
%D |
|---|---|---|
|
0.0 |
5.0 |
0.0 |
|
8.0 |
98.0 |
2.0 |
|
11.0 |
98.0 |
2.0 |
|
12.0 |
5.0 |
0.0 |
|
MS system: |
Xevo G2-XS QTof |
|
Ionization mode: |
ESI |
|
Acquisition range: |
50–1200 m/z |
|
Capillary voltage: |
3.0 kV |
|
Acquisition mode: |
MSE |
|
Resolution: |
30,000 FWHM |
|
MS software: |
MassLynx |
|
Informatics: |
Symphony, MSConvert, ElMaven, Polly |
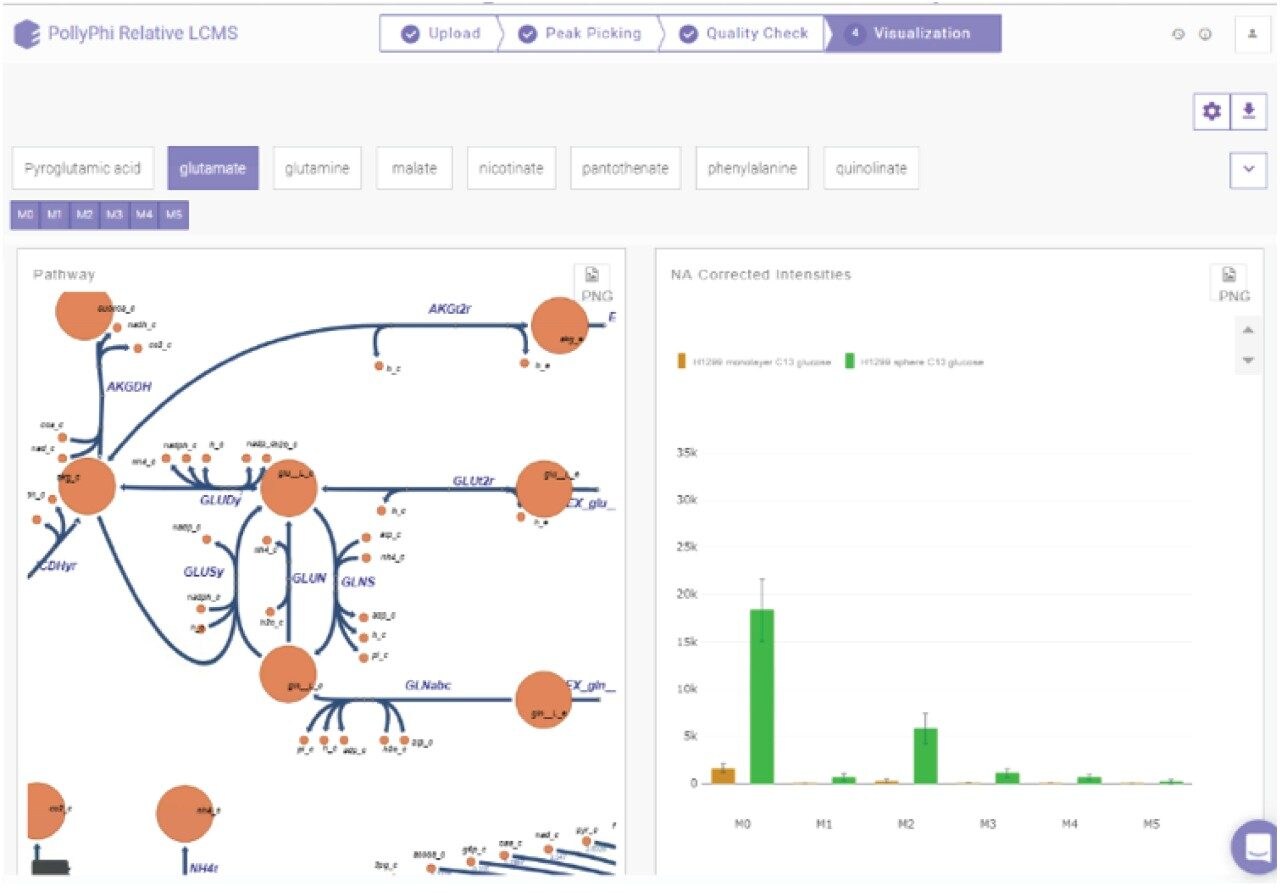
Figure 2 shows the basic components of a Symphony pipeline, a client/server application that is triggered by the MassLynx data acquisition system. Typically, a server request is executed that consists of a series of tasks that are executed based on input. Here, the pipeline transfers data and converts the native MassLynx format into mzXML using MSconvert (http://proteowizard.sourceforge.net/index.html). Next, it imports the converted data into ElMaven (elucidatainc.github.io/ElMaven) and Polly (elucidata.io) for peak detection, curation, natural abundance correction, and results visualization. In other words, mapping of the relative abundances and distribution of the detected metabolites on metabolic pathways as illustrated by the example shown in Figure 3. The details and benefits of the informatics pipeline have been described in detail elsewhere.4
The lactate fractional enrichment (13C3 isotopologue) is significantly higher in spheroid cell cultures compared to monolayer cell cultures. Since 13C3-lactate is the major isotopologue produced from 13C6-glucose, the primary energy source, the observations indicate that glycolysis is significantly upregulated in spheroid cell cultures as opposed to monolayers.
In addition, key intermediates in the tricarboxylic acid (TCA) cycle have higher 13C2 isotopologues. 13C2 isotopologues in the TCA cycle are generally formed from pyruvate 13C3 via acetyl-CoA through PDH enzyme. Synthesis of pyruvate 13C3 isotopologue can in turn be attributed to 13C6-glucose. This observation suggests a higher contribution of glucose to the TCA cycle via acetyl CoA in spheroid cell culture as opposed to monolayer cell culture. This is consistent with the finding of upregulated glycolysis in spheroid cells as shown and summarized in Figure 4.
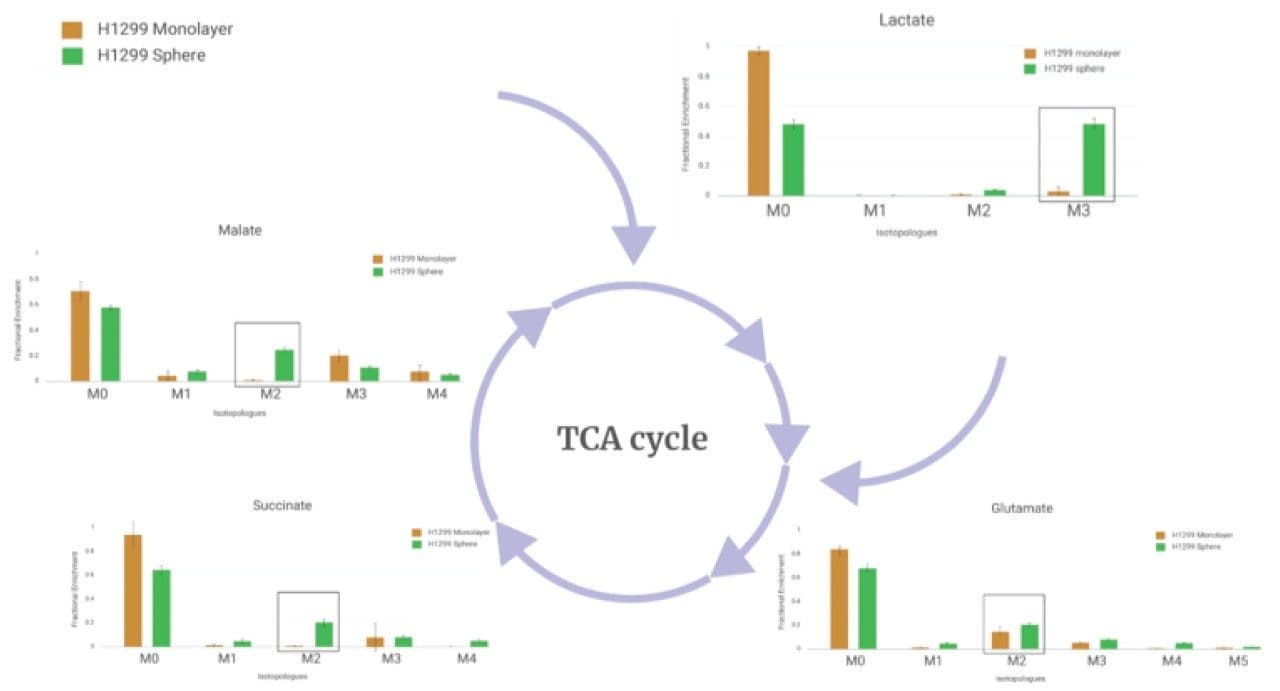
The direction of glucose in the same cell line can change dramatically depending on whether they are grown as 2D monolayers or 3D spheroids. Specifically, increased glucose flow through the glycolysis and TCA pathways were observed when they were grown as spheroids in contrast to monolayers. Such differences in nutrient utilization has significant implications for the translation of findings based on in vitro models to in vivo models. A prominent example has been shown in Figure 4 where the monolayer cell culture of H1299 human non-small cell lung carcinoma cells shows very limited glycolysis. In contrast, the observations and interpretations are very different for lactate. In the spheroid cells, glycolysis rates are increased significantly. Hence, analysis of the monolayer cell culture in isolation would have made translating the findings from the in vitro model to the in vivo model very challenging.
720006423, November 2018