
This work illustrates the robustness of the ionKey/MS System when analyzing complex biological samples produced by complex biological samples produced by common bioanalytical sample preparation techniques. We demonstrate the ionKey/MS System's robustness in the analysis of plasma samples prepared by crude protein precipitation (PPT), liquid-liquid extraction (LLE), and by immunoaffinity isolation followed by trypsin digestion (IA/TD).
This work illustrates the robustness of the ionKey/MS System when analyzing complex biological samples produced by common bioanalytical sample preparation techniques.
The robustness and reliability of pharmacokinetic (PK) data is an essential part of bioanalysis. LC-MS is the technique of choice in quantitative bioanalysis due to the high selectivity and sensitivity the technique offers. The use of reduced-bore chromatographic column dimensions such as 180–300 µm I.D. for the analysis of biological fluids can be traced back to the late 1990’s by Fraser et al. and has been shown as a means to increase assay sensitivity as well as reduce the amount of sample required to perform drug metabolism and pharmacokinetic (DMPK) analysis.1,2 However, the implementation of these scales of chromatography require: specialized equipment, smaller tubing I.D.s, and connections-related dead volumes in order to achieve the best chromatographic performance. Plugging of the small scale chromatographic components by the relative dirtiness of biological samples can be a major concern when implementing this scale of chromatography. In this work, we present the robustness in the utilization of the novel ionKey/MS System for analysis of plasma samples prepared by crude protein precipitation (PPT), liquid-liquid extraction (LLE), and by immunoaffinity isolation followed by trypsin digestion (IA/TD).
|
LC system: |
ACQUITY UPLC M-Class |
|
Separation device: |
iKey Peptide BEH 130Å, 1.7 μm, 150 μm x 50 mm (p/n 186006764) |
|
iKey temp.: |
40 °C |
|
Sample temp.: |
4 °C |
|
Injection vol.: |
1 μL |
|
Flow rate: |
4 μL/min |
|
Mobile phase A: |
0.1 % Formic acid in water |
|
Mobile phase B: |
0.1% Formic acid in Acetonitrile |
|
Gradient (PPT): |
5% B to 95% B over 5 minutes |
|
Gradient (LLE): |
5% B to 95% B over 5 minutes |
|
Gradient (IA/TD): |
0% B to 30% B over 5 minutes |
|
MS system: |
Xevo TQ-S |
|
Ionization: |
Positive ESI |
|
Acquisition mode: |
MRM |
|
Capillary voltage: |
3.2 kV |
|
Cone voltage and collision energies were optimized for each compound. |
|
Chromatography software: |
MassLynx 4.1 |
Human plasma was prepared by the addition of acetonitrile in a ratio of 2:1 (acetonitrile:plasma). The plasma sample was then vortex mixed for one minute and subsequently centrifuged at 5,000 relative centrifugal force (RCF) for five minutes. The supernatant was then removed, pipetted into an LC vial, and injected onto the LC-MS system. At regular intervals of fifty injections, a QC standard, consisting of dextromethorphan and propranolol, was monitored to access chromatographic performance over the test period.
Human plasma was prepared by the addition of hexane in a ratio of 10:1. The plasma sample was then vortex mixed for one minute and subsequently centrifuged at 5,000 RCF for five minutes. The supernatant was then removed into a new vial. The sample was then dried down and reconstituted in one fifth the initial volume, and injected onto the LC-MS system. At regular intervals of twenty injections, a QC standard, consisting of dextromethorphan and propranolol, was monitored to access chromatographic performance over the test period.
Samples were kindly obtained from Bristol Myers Squibb (BMS). Human plasma was spiked with a therapeutic monoclonal antibody (mAb) and immunoaffinity isolation, implemented in the magnetic bead format, was used for the isolation of the mAb from plasma. After denaturation, the mAb was digested with trypsin. Over 1,000 injections of the mAb digest were performed and two signature peptides were monitored in each LC-MS run to evaluate the chromatographic performance over the test period.
Routine analysis within a bioanalytical laboratory usually consists of a batch of two to four 96-well sample plates that have been prepared by the bench chemist using protein precipitation, liquid-liquid extraction, or in the case of biopharmaceuticals such as monoclonal antibodies, immunoaffinity isolation followed by proteolytic digestion. Of these techniques, protein precipitation is the most commonly utilized due to the speed and relative low cost of the technique for the analysis of small molecules.3 However this technique is also the crudest in its ability to produce clean samples for analysis. Because of this fact, robustness testing of a novel 150 µm iKey Separations Device was carried out under these conditions as they provided the most challenging of the samples preparation techniques of biological fluids. The ionKey/MS System, comprised of the Xevo TQ-S Mass Spectrometer, the ACQUITY UPLC M-Class, the ionKey Source, and the iKey Separation Device is shown in Figure 1. The iKey Separation Device consists of the ceramic-based separations device with an integrated emitter together in a single device that is placed directly into the source of the mass spectrometer.

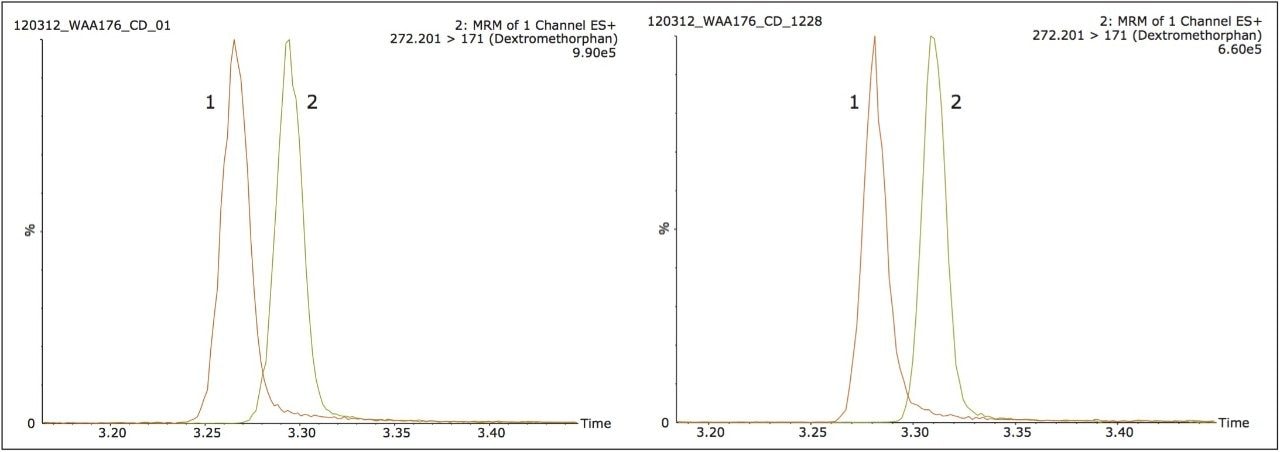
Figure 2 illustrates the separation of dextromethorphan and propranolol, during a generic five minute LC gradient. Here we observe a peak width at 10% height for dextromethorphan and propranolol of one second. The calculated resolution at this peak height was 0.8. In Figure 2 we again show the injection of the QC standard after 1,000 injections (5 days) of continuous 1 µL injections of the protein precipitated human plasma. It should be noted that a 1 µL injection on the 150 µm I.D. iKey Separation Device is equivalent to 200 µL on a traditional 2.1 mm I.D. analytical column. The comparison of the data shown in Figure 2 indicates that excellent peak symmetry and chromatographic resolution of 0.9 were maintained over the course of the testing period.
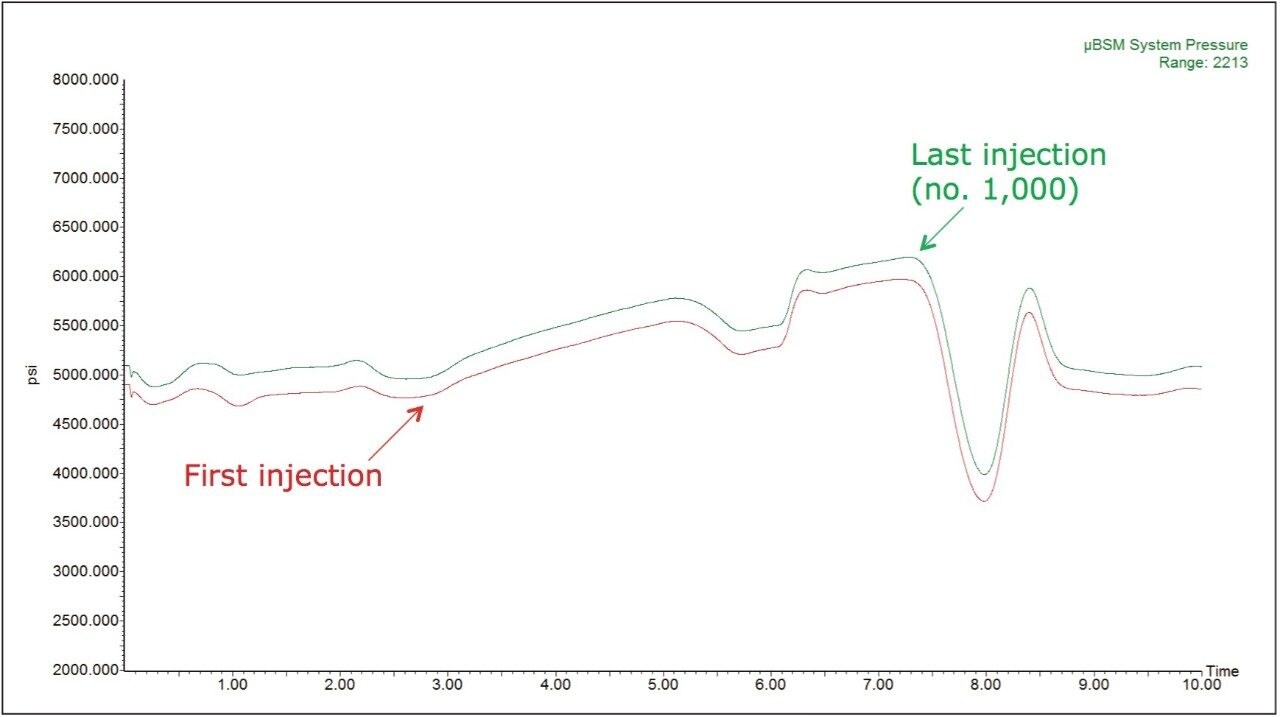
The system pressure traces from the study are shown in Figure 3. Here we observe no discernible increases in the system pressure, indicating that none of the frits, tubing or connective fittings have been blocked over the course of the study. It should again be noted that this injection volume of 1 µL is analogous to injecting roughly 200 times this volume or roughly 200 µL onto a standard 2.1 mm I.D. column. Cleanliness of the MS source is often a key parameter in the continued acquisition of quality data over the course of a study. The inherent ability in the use of smaller volumes to achieve similar sensitivity results as with standard 2.1 mm I.D. scale LC-MS equates to less contamination of the MS source from the sample.
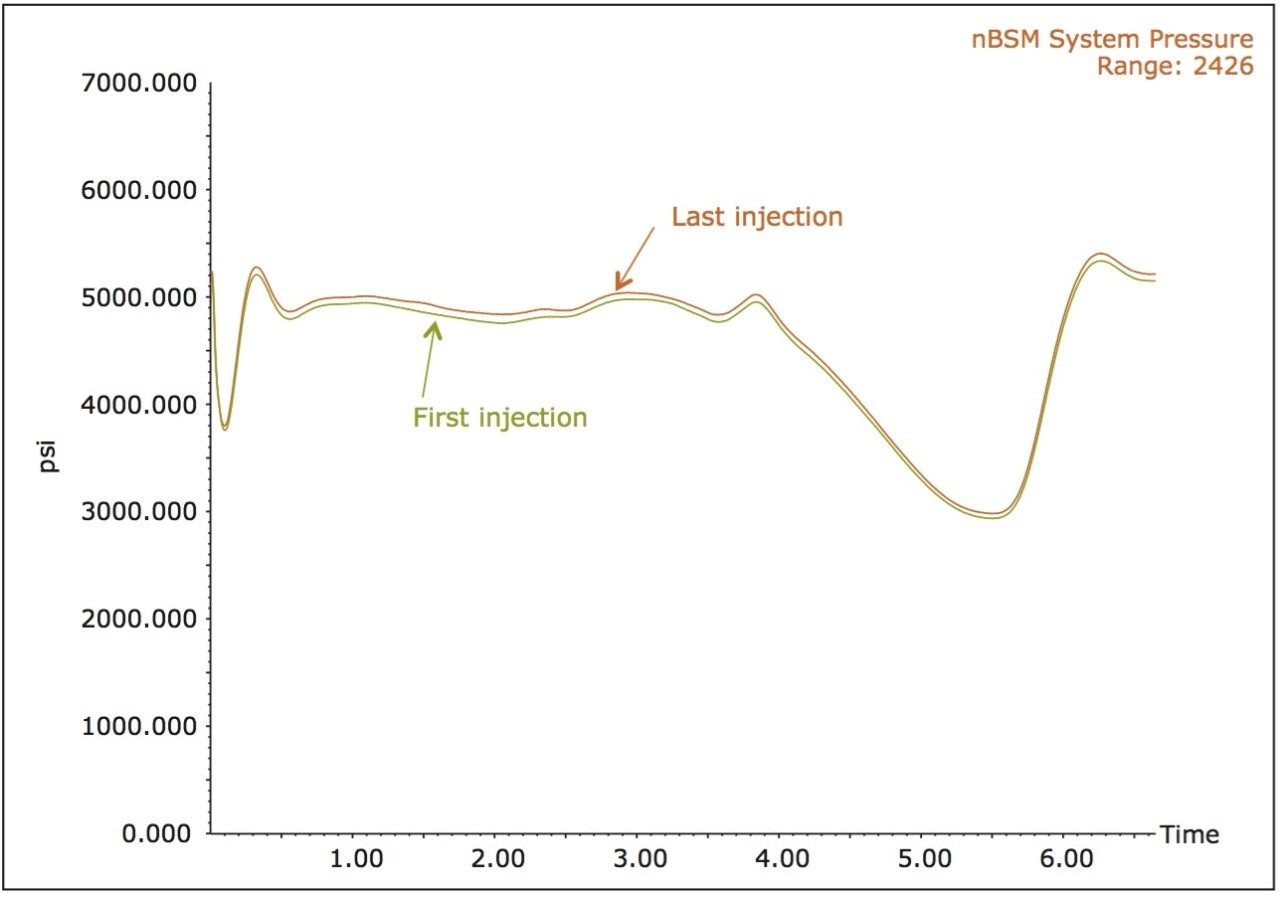
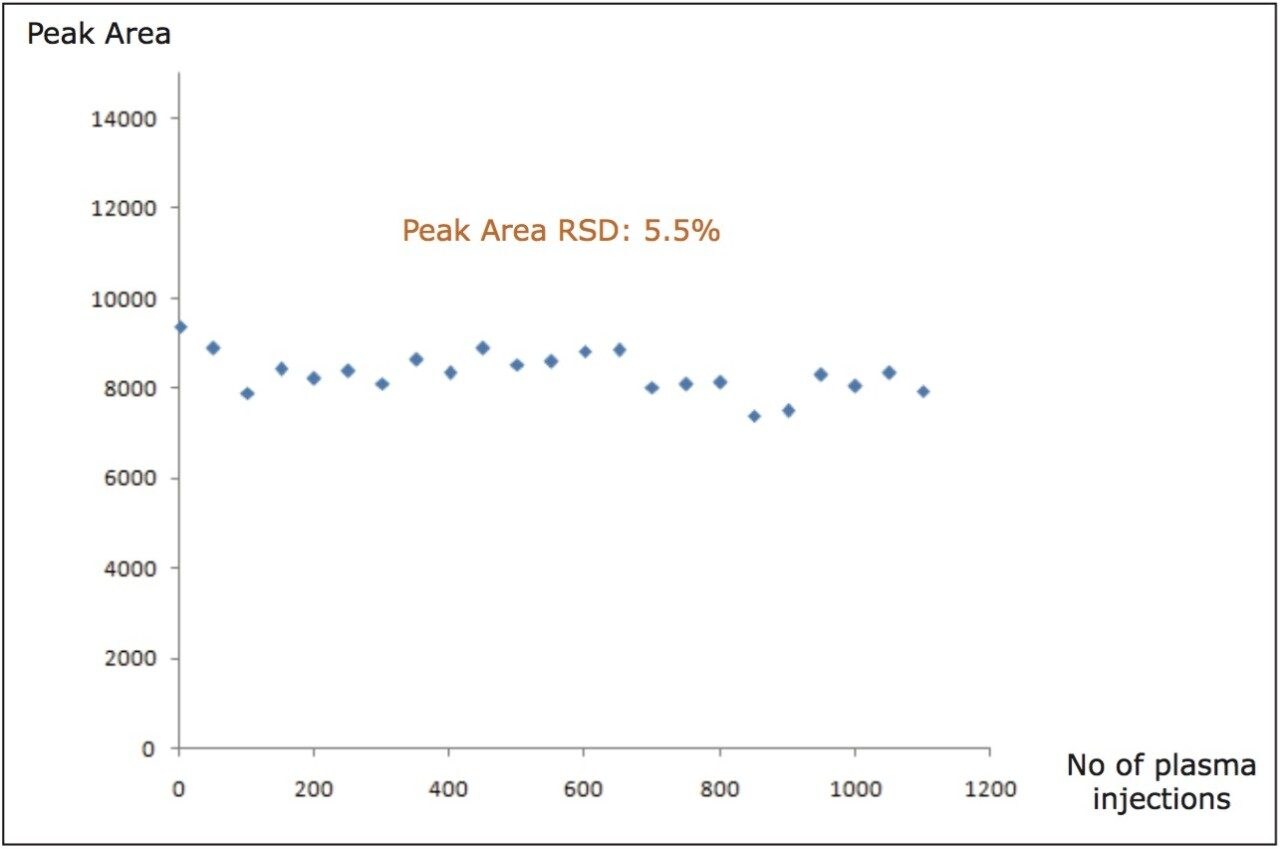
Figure 4 illustrates the stability of the peak area from the QC sample over a course of 1000 injections of the protein precipitated human plasma sample. This data further illustrates the robustness, not only of the iKey Separations Device, integrated emitter, MS source but the entire system. The system was next challenged with human plasma samples prepared by LLE. This test utilized the same experimental conditions as with the previous example. Figure 5 shows the QC standard of propranolol and dextromethorphan at injection 1 and injection 1000 and illustrates that both the chromatographic peak shape and the resolution where maintained over the course of the study, much in the same manner as with the previous example. It should be further noted that again no discernible increase in system backpressure was observed.
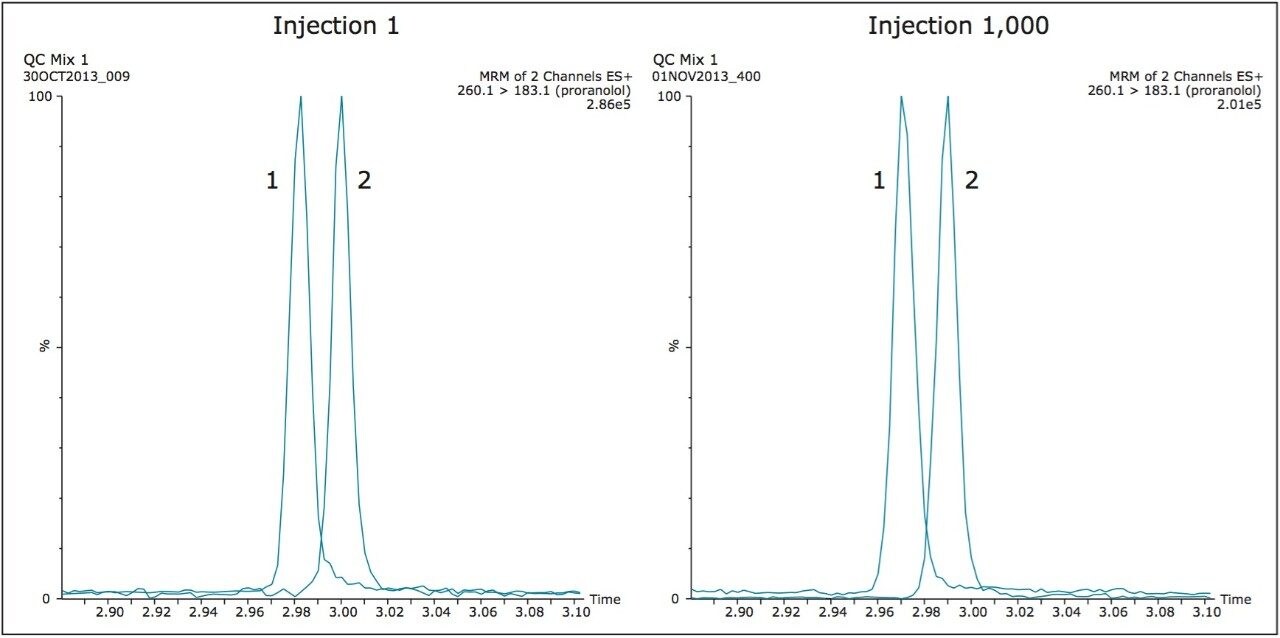
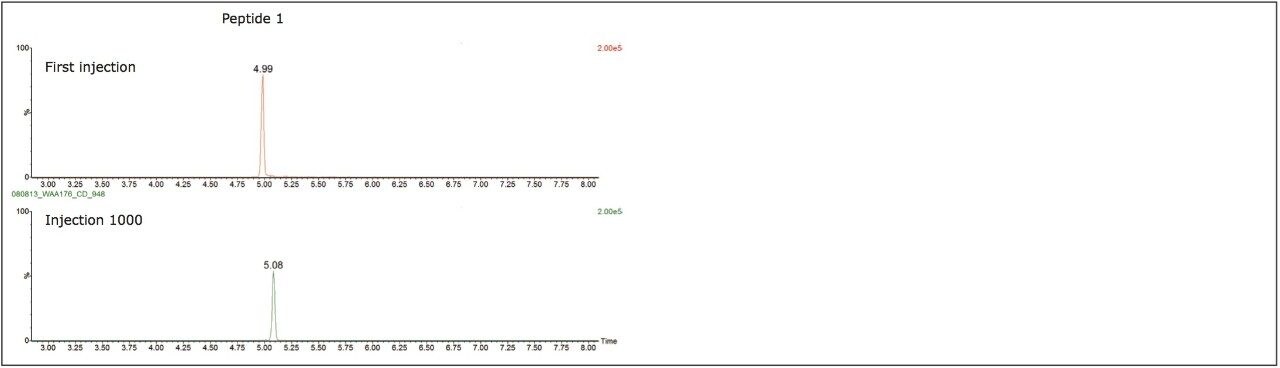
Due to the increase in the development of biopharmaceuticals, such as antibody therapeutics, the robustness of the ionKey/MS System was tested with a common sample preparation scheme of affinity isolation followed by digestion and subsequent analysis of peptides generated from the sample preparation. As with the previous studies chromatographic performance and system pressure where monitored. In this example, specific peptides produced by the trypsin digestion of the antibody where monitored over the course of the study. Figure 6 illustrates the peak shape at injection 1 and injection 1,000 for two of the signature peptides monitored during the study. The typical peptide peak widths (at 10% peak height) observed under the experimental conditions employed were 3–4 seconds.
As in the previous experiments, pressure traces were recorded throughout the entire study and the first and the last pressure traces are displayed in Figure 7.
The performance of the ionKey/MS System, using a novel 150 µm I.D. iKey Separation Device packed with 1.7 µm chromatographic particles, in the analysis of biological fluids was shown to be:
Waters would like to thank Eugene Ciccimaro, Bogdan Sleczka, Celia D’Arienzo, and Timothy Olah from BMS for the production and use of the digested antibody samples utilized in this study.
720004953, February 2016