For forensic toxicology use only.
This is an Application Brief and does not contain a detailed Experimental section.
This Application brief demonstrates the performance of 1.6 μm CORTECS Phenyl Columns for forensic toxicology applications.
As LC-MS/MS continues to grow as a forensic screening and quantification technique, high efficiency columns with appropriate selectivities are needed to address the demands of modern laboratories.
Analysis of broad toxicological panels by LC-MS/MS continues to grow as many laboratories replace immunoassay based screening methods, GC-MS methods, update older LC-MS methods, or simply consolidate multiple analyses into single panels.1 While many reversed-phase columns have been used for these applications, Waters has developed a solid-core particle bonded with a phenyl functionality. This combination has resulted in a high efficiency column with unique selectivity that can be used to analyze a wide variety of forensically relevant drugs.
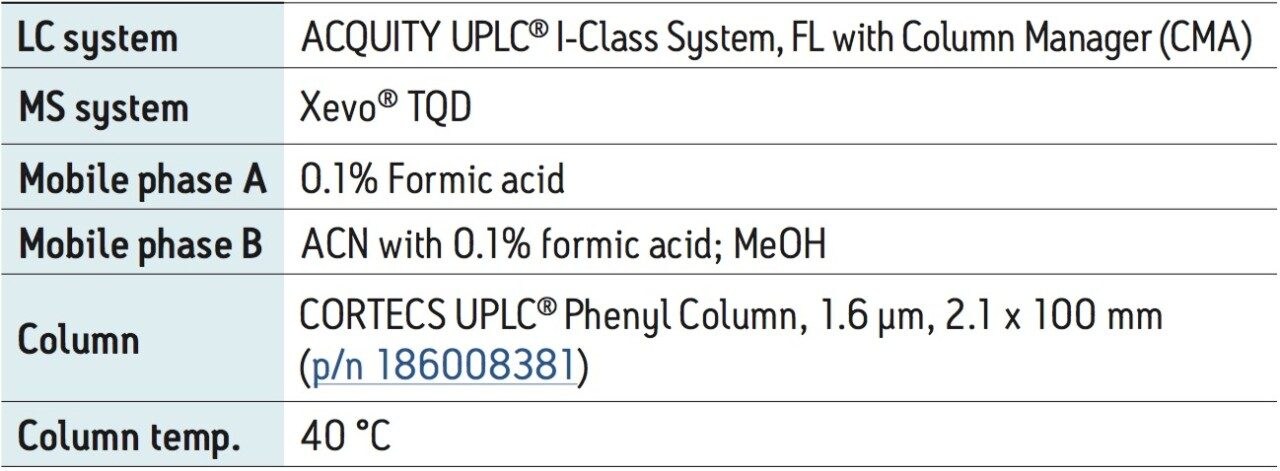
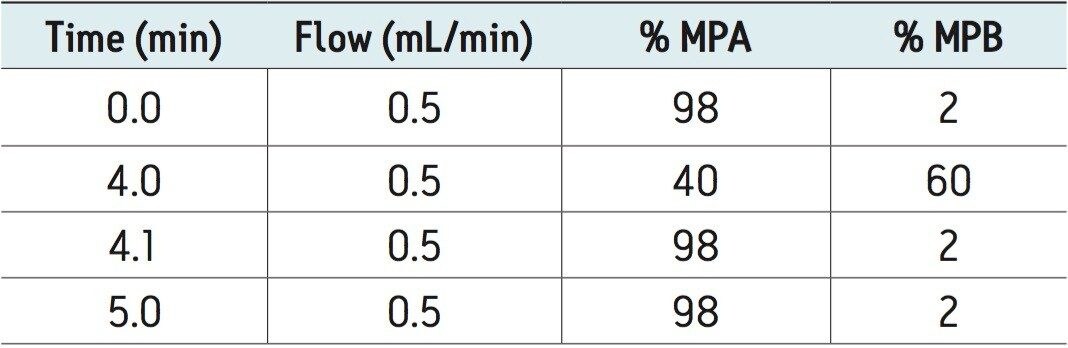
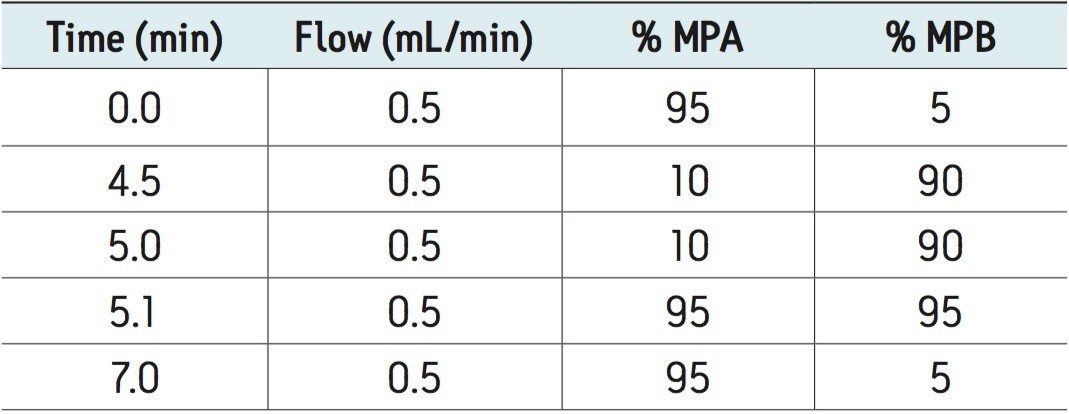
UPLC-MS/MS methods were developed for a panel of forensically relevant drugs that include natural and synthetic opioids and their metabolites, benzodiazepines, amine stimulants, PCP, and benzoylecgonine (BZE). Chromatographic separations were conducted using both acetonitrile (ACN) and methanol (MeOH) as organic mobile phases. This approach was taken to demonstrate differential column performance using the two most popular organic mobile phases. The use of methanol maximizes the pi-pi bonding interactions between the phenyl stationary phase and the aromatic rings of many of the compounds of interest, resulting in different selectivity compared to acetonitrile. The general method parameters are listed in Table 1 and the chromatographic conditions for each mobile phase combination are listed in Tables 2A and 2B.
Both methods were rapid with all compounds eluting in under 4.5 minutes. These separations were also highly efficient. Average peak widths for ACN and MeOH were 2.41 and 3.00 seconds, respectively. In each case, baseline resolution was achieved for all isobaric compounds, such as morphine and hydromorphone, as well as structural isomers with the same molecular mass, such as methamphetamine and phentermine. Differential selectivity can be seen for several compounds. For example, benzoylecgonine (compound 22) demonstrates a significant shift to a later retention time relative to other compounds when methanol is used vs. acetonitrile (see Figures 1A and 1B). A selectivity difference can also be seen with methamphetamine and phentermine, which have the same molecular mass and share an MRM transition. While baseline separated using acetonitrile (1.47 vs 1.52 min.), the use of methanol significantly improves their separation from 3.0 to 9.6 seconds, which translates to a resolution increase from 1.88 to 4.85, a significant enhancement for two compounds than can interfere with each other. The benzodiazepines also demonstrate selectivity differences between the two mobile phases. Flunitrazepam and temazepam coelute with ACN but are baseline separated when using MeOH. A similar change is seen with oxazepam and nitrazepam, whose retention time difference changes from .02 minutes to .08 minutes.
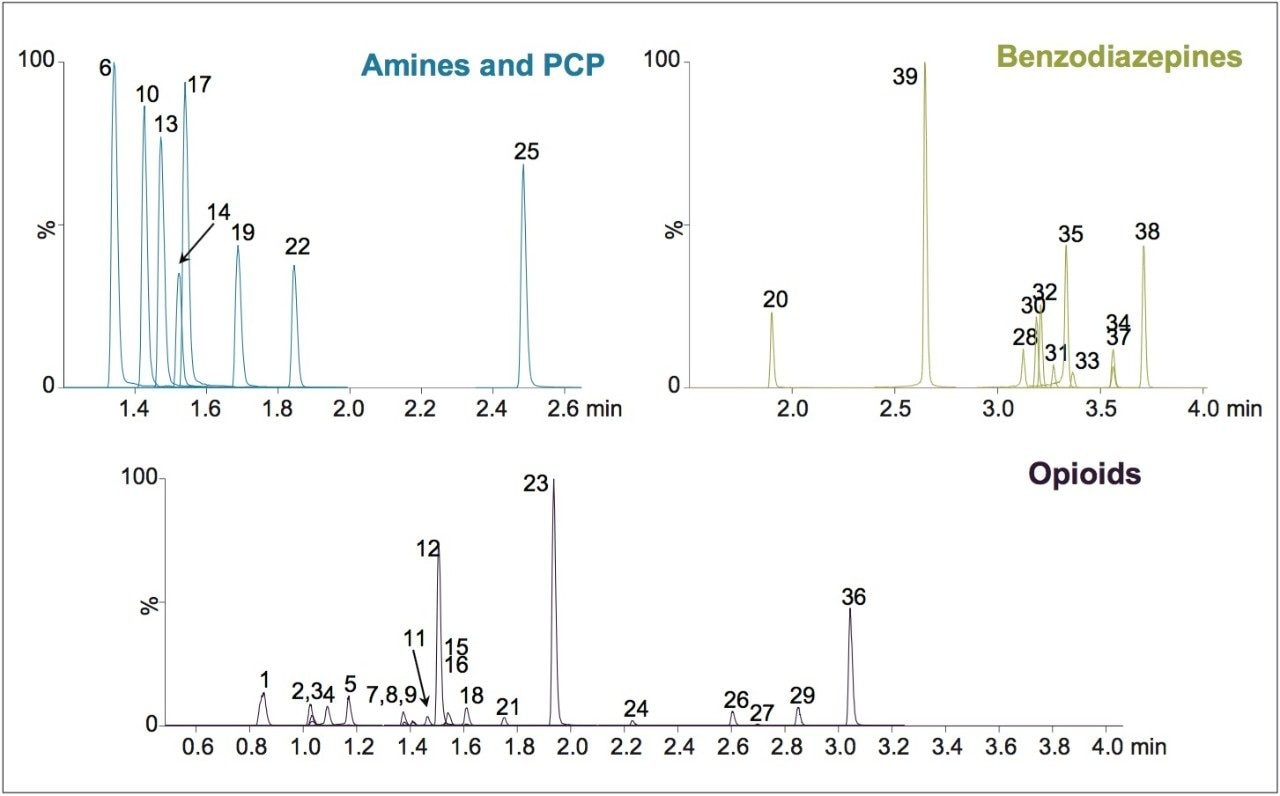
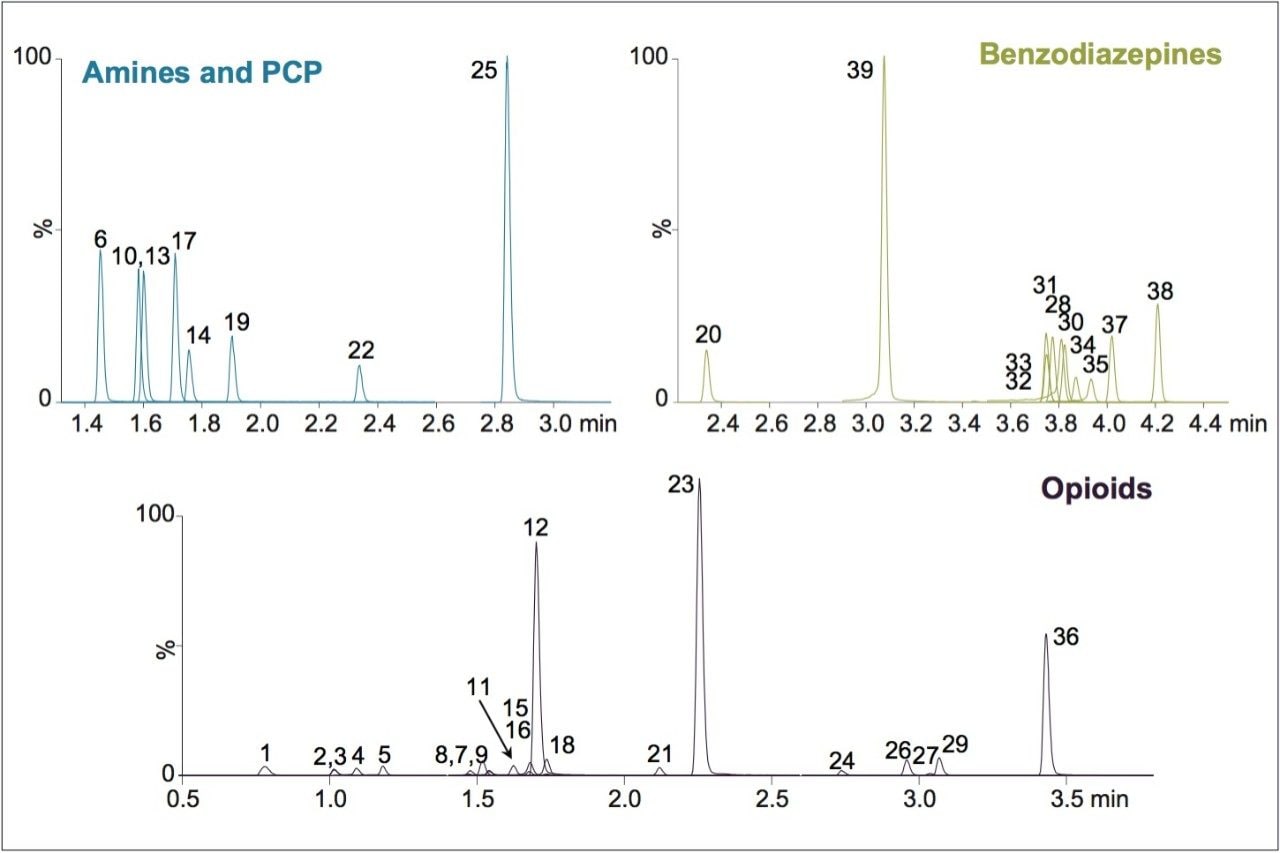
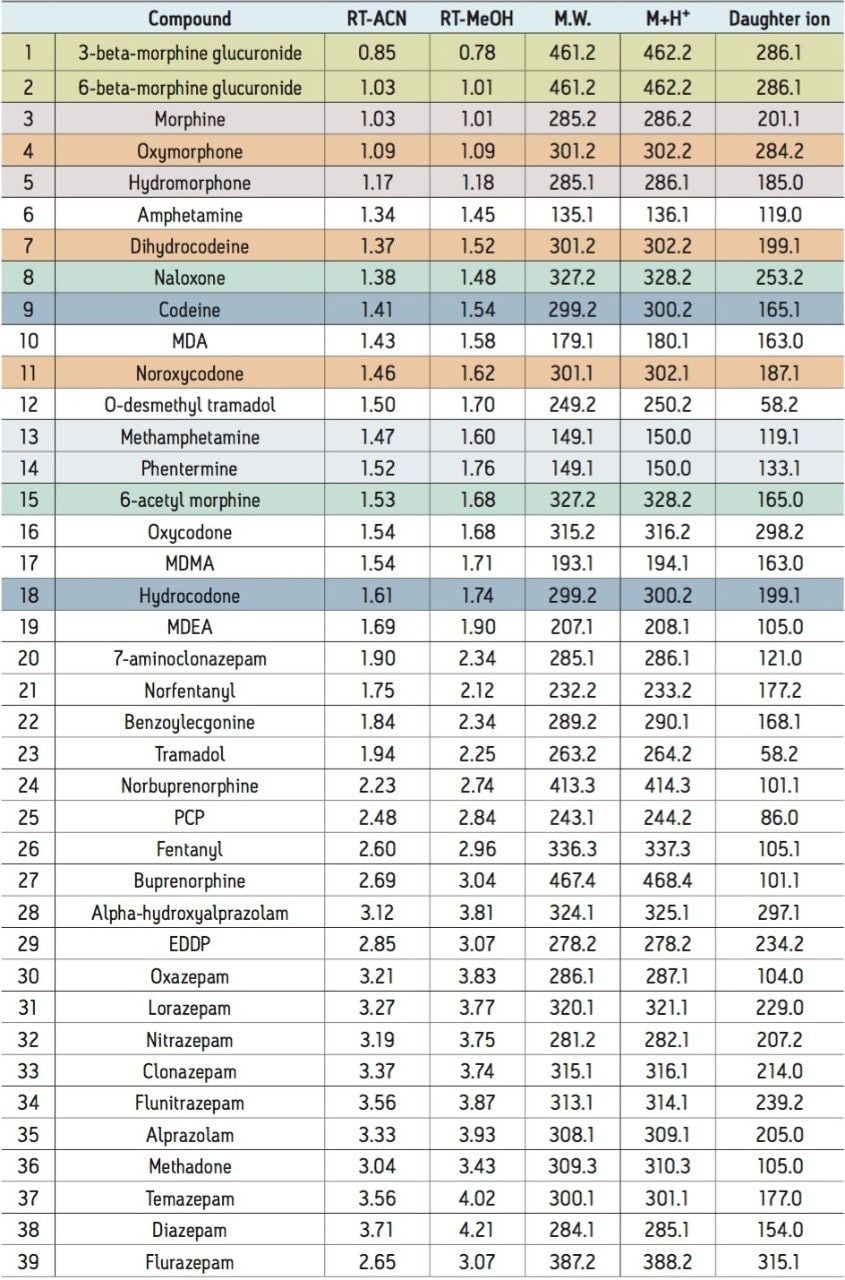
We have developed UPLC-MS/MS methods for a panel of forensically relevant drugs using the new CORTECS UPLC Phenyl Column, 1.6 μm, 2.1 x 100 mm (p/n 186008381). Separations were highly efficient and all compounds with the potential to interfere with each other were baseline separated. The use of different organic mobile phases revealed significant selectivity differences that can be exploited, if necessary, to optimize separation of critical analyte pairs. This column represents the continued development of the CORTECS Solid-Core Column family.
720005569, January 2016