
The USP compendial method for donepezil tablets was successfully transferred from HPLC to UPLC using the Waters Column Selectivity Chart and ACQUITY UPLC Columns Calculator. Routine use, typical of a QC laboratory analyzing a formulated donepezil tablet sample, was evaluated on the ACQUITY UPLC BEH C18 1.7 μm column. Approximately 850 injections were achieved before peak splitting was evident and the method failed system suitability. Troubleshooting steps attributed the cause of failure to sample build-up on the column bed. Due to practical limitations in changes to the sample preparation, the method was transferred to an XBridge C18 XP 2.5 μm column, where approximately 1600 injections were achieved. The method using the XP 2.5 μm column is approximately 80% faster than the HPLC method and results in a 92% savings in sample injected and solvent consumption. In cases where formulated sample is not directly compatible with the original compendial method, the use of XP 2.5 μm columns can provide method ruggedness while still providing significant sample throughput and solvent cost savings.
Compendial methods are often used in the analysis of generic drugs. Typically, methods are developed and submitted based on a specific formulation. However when the excipients are changed, the method specified in a USP monograph may be inadequate in eluting all components off of the column. The result can be deteriorating peak shape and premature method failure due to a build-up of poorly soluble excipients on the column.
Donepezil is a drug used to treat symptoms of dementia in Alzheimer’s patients and is formulated in a tablet preparation. Here, we demonstrate the transfer of the USP compendial method for donepezil from HPLC to UPLC. Methods for assay and impurities analyses are sometimes combined to minimize the number of methods developed and submitted for regulatory approval. In this application, the transfer of the organic impurities method, which is a more complicated gradient separation, is demonstrated. Since impurity standards were not readily available, the transfer and routine use study is demonstrated using donepezil standard and tablets. A routine use study using conditions typical of a QC laboratory protocol is performed with the UPLC method and assay suitability criteria are evaluated to assess the long-term robustness of the method.
|
Column: |
XBridge C18, 4.6 x 250 mm, 5 μm (L1), part number 186003117 |
|
Gradient: |
25% B to 60% B over 10 min, hold for 30 min, return to 25% B in one min and re-equilibrate for 9 min |
|
Flow Rate: |
1.5 mL/min |
|
Run Time: |
50 min |
|
Injection Volume: |
20 μL |
|
Column: |
ACQUITY UPLC BEH C18 2.1 x 100 mm, 1.7 μm (L1), part number 186002352 |
|
Gradient: |
25% B to 60% B over 2.5 min, hold for 7.5 min, return to 25% B in 0.25 min and re-equilibrate for 2.25 min |
|
Flow Rate: |
0.5 mL/min |
|
Run Time: |
12.5 min |
|
Injection Volume: |
1.7 μL |
|
Column: |
XBridge C18 XP, 2.1 x 100 mm, 2.5 μm (L1), part number 186006031 |
|
Gradient: |
25% B to 60% B over 2 min, hold for 6 min, return to 25% B in 0.2 min and re-equilibrate for 1.8 min |
|
Flow Rate: |
0.63 mL/min |
|
Run Time: |
10 min |
|
Injection Volume: |
1.7 μL |
|
Mobile Phase: |
A: 0.1% phosphoric acid in water, adjust to pH 6.5 with triethylamine B: acetonitrile |
|
Diluent: |
water:acetonitrile (3/1) |
|
Needle Wash: |
60:40 water:acetonitrile |
|
Sample Purge: |
60:40 water:acetonitrile |
|
Seal Wash: |
50:50 methanol:water |
|
Column Temp.: |
50 °C |
|
Detection: |
UV at 286 nm |
|
Data Management: |
Empower 3 CDS |
|
Tailing Factor: |
Not More Than (NMT) 1.5 USP Plate Count: Not Less Than (NLT) 40,000 plates |
|
Replicate Injections: |
NMT 2.0% RSD for donepezil peak (5 replicates) |
Donepezil (1 mg/mL) in diluent: Waters Analytical Standard
Donepezil Tablets (1 mg/mL): Crushed tablets were weighed in a 50 mL volumetric flask and 25 mL diluent was added. The sample was sonicated for 15 minutes and made up to volume with diluent. The sample was mixed well, filtered though a 0.2 µm PTFE filter and centrifuged at 12,000 rpm for 5 minutes.
The USP method for donepezil describes sample preparation as ‘dissolve in diluent and sonicate if necessary’. When preparing the tablet sample according to the USP method, the final product is a solution with visible fine particulates. In order to render the sample clear and suitable for injection, the sample was further filtered through a 0.2 μm PTFE membrane and then centrifuged at 12,000 rpm for 5 minutes. Upon centrifugation, a small pellet was visible and the supernatant was carefully pipetted into a sample vial for injection.
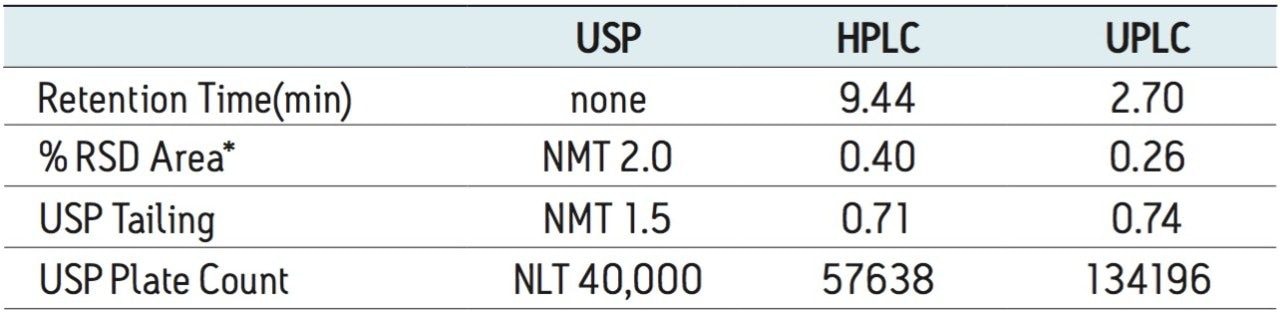
The USP organic impurities method for donepezil describes the use of an L1 column and the suggested column is Kromasil C18. Using the Waters Column Selectivity Chart, a more modern L1 column, XBridge C18, was selected since this column chemistry is also available in smaller particle sizes. The USP compendial method was first run as described on an Alliance HPLC system using five replicate injections of donepezil standard and donepezil prepared tablet. System suitability criteria described in the monograph were monitored and found to be within specifications (Table 1).
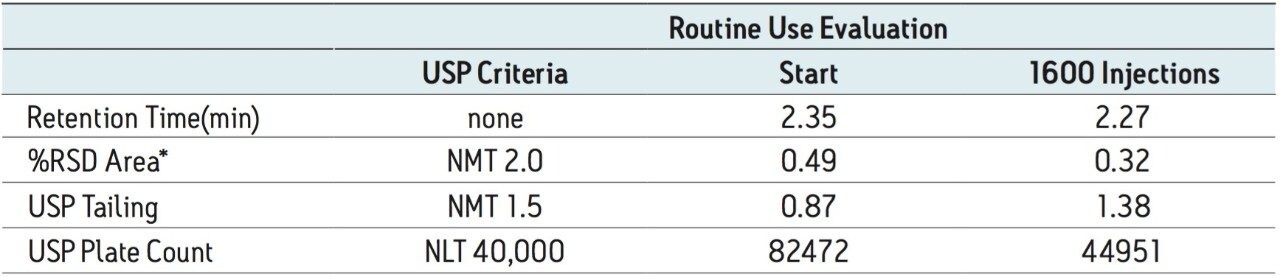
The USP method was then transferred from HPLC to UPLC using the ACQUITY UPLC Columns Calculator. Scaling was performed accounting for particle size and the column was scaled to an ACQUITY UPLC BEH C18 1.7 μm column, which has the identical stationary-phase chemistry to the HPLC column (XBridge C18). Five replicate injections of both donepezil standard and donepezil tablet were analyzed separately. System suitability criteria for donepezil, including %RSD for peak area, USP tailing and USP plate count were compared between HPLC and UPLC. A comparison of both systems is shown in Table 1, where the UPLC transferred method passes all criteria specified in the monograph. The run time of the UPLC method is 12.5 minutes compared to the 50-minute HPLC method (Figure 1), resulting in a 75% savings in analysis time and 92% savings in solvent consumption and sample injected.
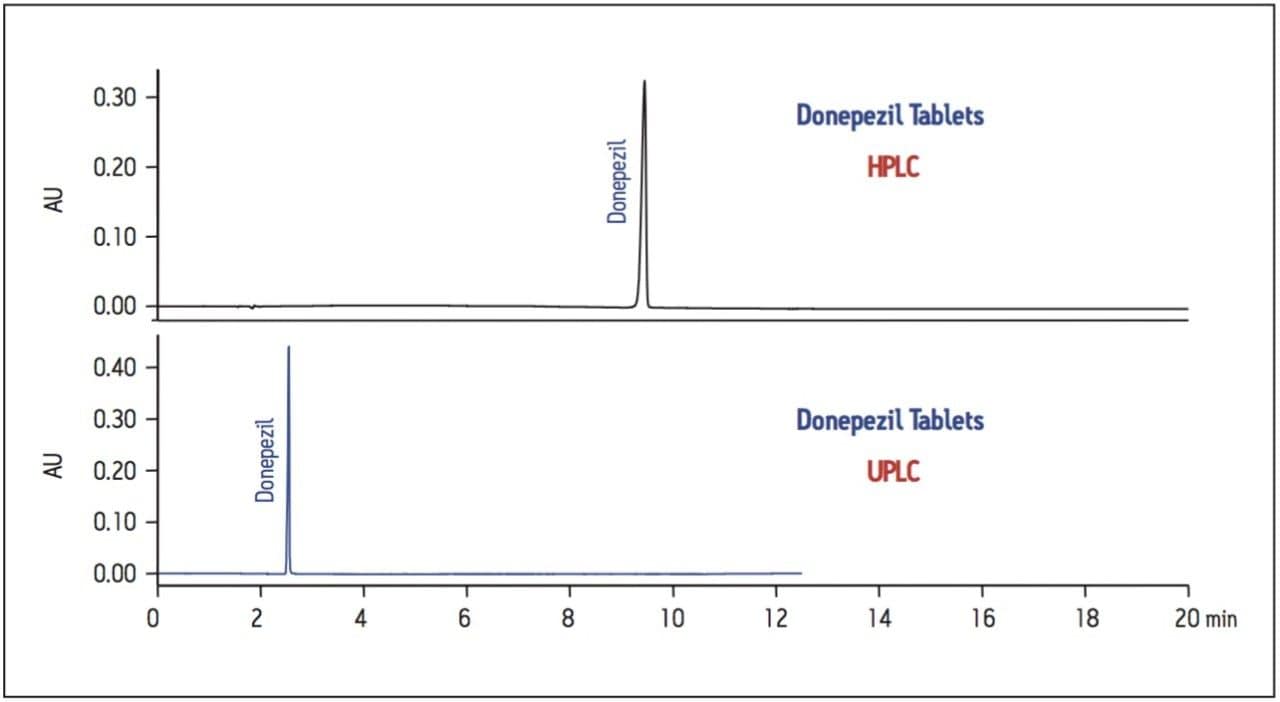
Evaluation #1
In order to evaluate the effects of repeatedly analyzing a tablet drug formulation using a UPLC transferred USP method, a routine use evaluation was performed with the ACQUITY UPLC BEH C18, 1.7 μm column using donepezil tablets.
Donepezil tablet samples were injected repeatedly using donepezil standard as a bracketing standard to emulate the process in a typical quality control (QC) laboratory. Mobile phase was prepared fresh daily to prevent bacterial growth. Twenty injections of donepezil tablet samples were made, along with five replicate injections of donepezil standard, and this cycle was repeated continuously until assay suitability criteria no longer passed. Pressure, retention time, peak area, USP tailing and USP plate count were monitored throughout the study.
USP tailing remained within assay criteria throughout the study, however, after approximately 700 injections, the USP plate count began rapidly decreasing (Figure 2). At approximately 850 injections, the donepezil peak shape began to split (Figure 3) and the routine use study was stopped. Pressure remained stable throughout the study. The system was cleaned, mobile phases replaced and the column was washed. The column inlet and outlet frits were also replaced to determine if the poor peak shape could be attributed to particulate contamination, but the USP plate count still did not pass system suitability. These troubleshooting measures indicated that the UPLC column stationary phase had become contaminated by the poorly soluble excipients in the donepezil tablet formulation.

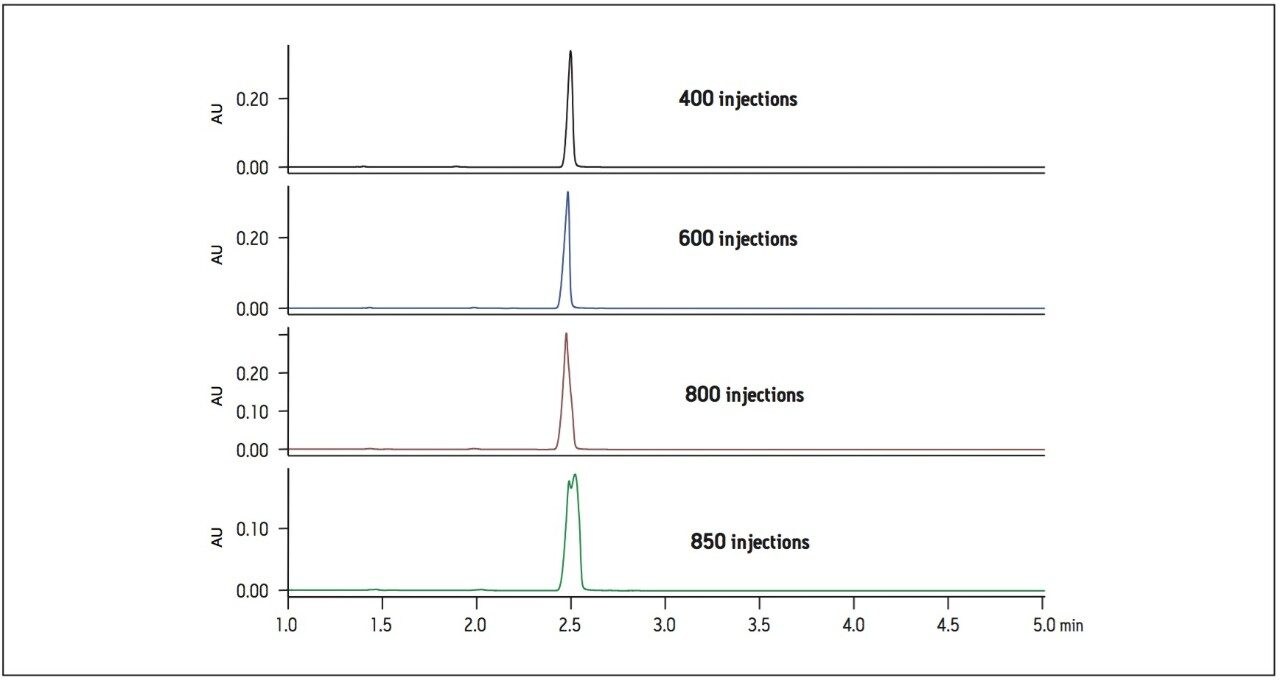
Sample preparation for formulated drug tablets is often limited due to time, cost and recovery concerns. For this reason, additional sample preparation steps were not pursued. Instead, the UPLC method using the ACQUITY UPLC 1.7 μm column was transferred to an XBridge C18 XP 2.5 μm column using the ACQUITY UPLC Columns Calculator. Due to the larger particle size and subsequently lower backpressure, the method was run at a higher linear velocity, which resulted in a 10-minute method, an 80% savings in analysis time compared to the original 50-minute HPLC method.
A second routine use study was performed with the same repeating sample set containing donepezil standards and tablets, using the XP 2.5 μm column. The results of this routine use study show USP plate count and USP tailing factor failing criteria after about 1600 injections (Figure 4). The routine use study was stopped at approximately 1700 injections, when the peak shape deterioration became evident (Figure 5).
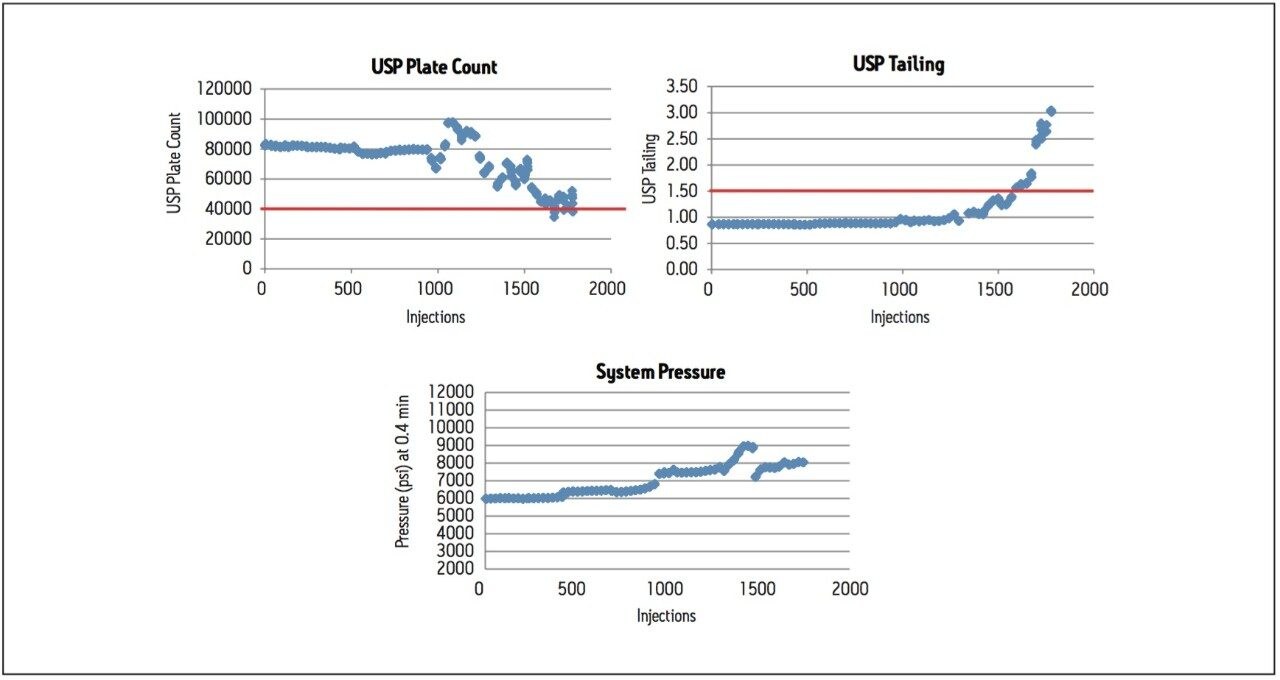
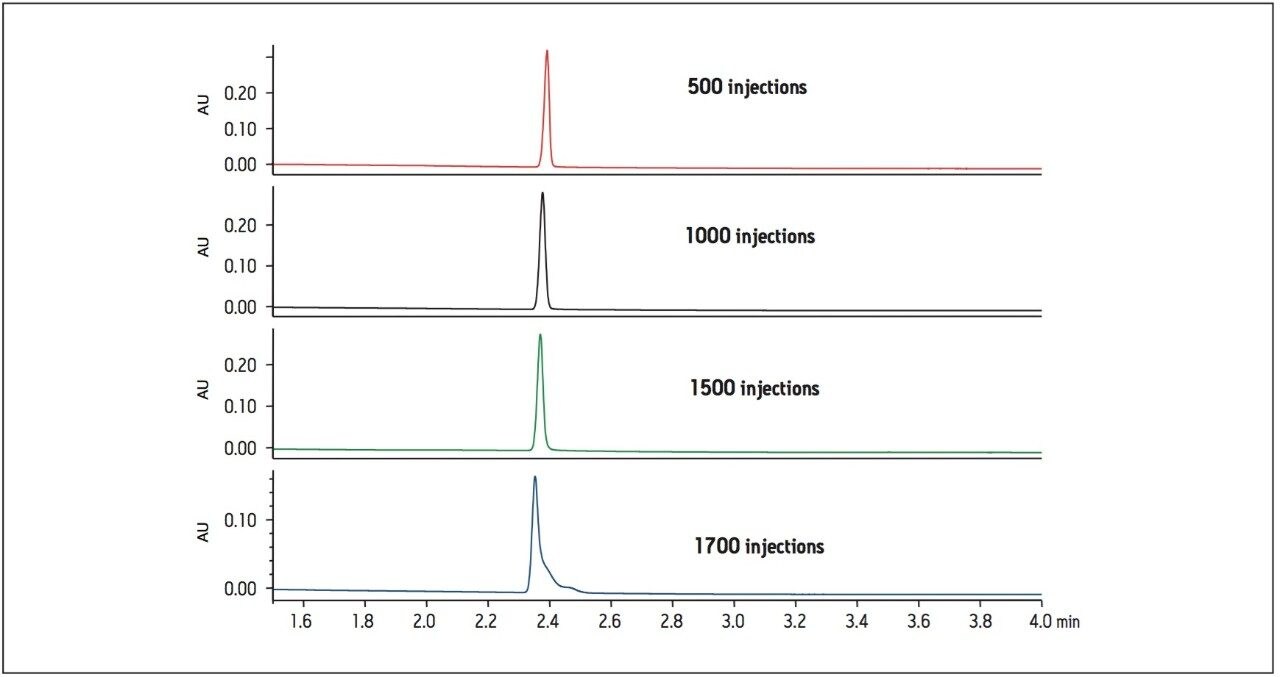
Although the same formulation was analyzed, the XP 2.5 μm column resulted in twice as many injections compared to the 1.7 μm column. At 1600 injections, all system suitability criteria were still within specification (Table 2). In the analysis of complex formulation matrices, there is limited removal of insoluble excipients using simplistic sample preparation procedures. This often results in an unavoidable build-up of sample components on the column. In the case of donepezil tablets, the use of an XP 2.5 μm column allowed for a greater number of injections, while still meeting the USP monograph system suitability requirements.
The USP compendial method for donepezil tablets was successfully transferred from HPLC to UPLC using the Waters Column Selectivity Chart and ACQUITY UPLC Columns Calculator. Routine use, typical of a QC laboratory analyzing a formulated donepezil tablet sample, was evaluated on the ACQUITY UPLC BEH C18 1.7 μm column. Approximately 850 injections were achieved before peak splitting was evident and the method failed system suitability. Troubleshooting steps attributed the cause of failure to sample build-up on the column bed. Due to practical limitations in changes to the sample preparation, the method was transferred to an XBridge C18 XP 2.5 μm column, where approximately 1600 injections were achieved. The method using the XP 2.5 μm column is approximately 80% faster than the HPLC method and results in a 92% savings in sample injected and solvent consumption. In cases where formulated sample is not directly compatible with the original compendial method, the use of XP 2.5 μm columns can provide method ruggedness while still providing significant sample throughput and solvent cost savings.
720004261, March 2013