This study details a cleaner, more reproducible extracts than competitive phospholipid removal devices or techniques, Ostro allows for more robust analyses and increased sample throughput. Requiring minimal to no method development, this technology can be rapidly implemented in order to improve laboratory workflow.
Matrix effects are residual components of biological matrix that alter mass spectrometry response, decrease method robustness, add to method variability, and lead to poor limits of detection/quantification. A major source of matrix effects in plasma-based bioanalytical assays is residual phospholipids. If not removed from the sample, phospholipids can build up on analytical columns, elute at unpredictable times in subsequent injections, and negatively impact assay robustness in a number of different ways. Over the past few years, it has become commonplace to monitor phospholipids during method development and to implement strategies to remove them from plasma samples. Sample preparation strategies for phospholipid (PL) removal include the use of specific PL removal plates, liquid-liquid extraction (LLE), and solid-phase extraction (SPE). During the method development process, decisions are made regarding the choice of a “fit-for-purpose” sample prep technique. Ostro Sample Preparation Products are designed to provide a novel solution for the rapid and simple cleanup of phospholipids in biological samples prior to LC-MS/MS analysis. Using a convenient 96-well format, in-well protein precipitation is performed with a single pass through method which provides consistent, high quality results, including significant removal of phospholipids for cleaner extracts, optimal recovery for diverse analytes, and increased reproducibility for consistent, robust methods.
|
LC system: |
Waters ACQUITY UPLC System with a Waters Xevo TQ MS triple quadrupole mass spectrometer operated in positive ion MRM mode |
|
Column: |
ACQUITY UPLC BEH C18, 2.1 x 50 mm, 1.7 μm |
|
Injection volume: |
8 μL |
|
Flow rate: |
0.6 mL/min |
The Ostro 96-well plate was directly compared to protein precipitation (PPT), 2 competitive phospholipid removal plates, LLE and solid-supported liquid-liquid extraction (SSLE.) Analyte recovery, phospholipid removal, and reproducibility of phospholipid removal were all evaluated.
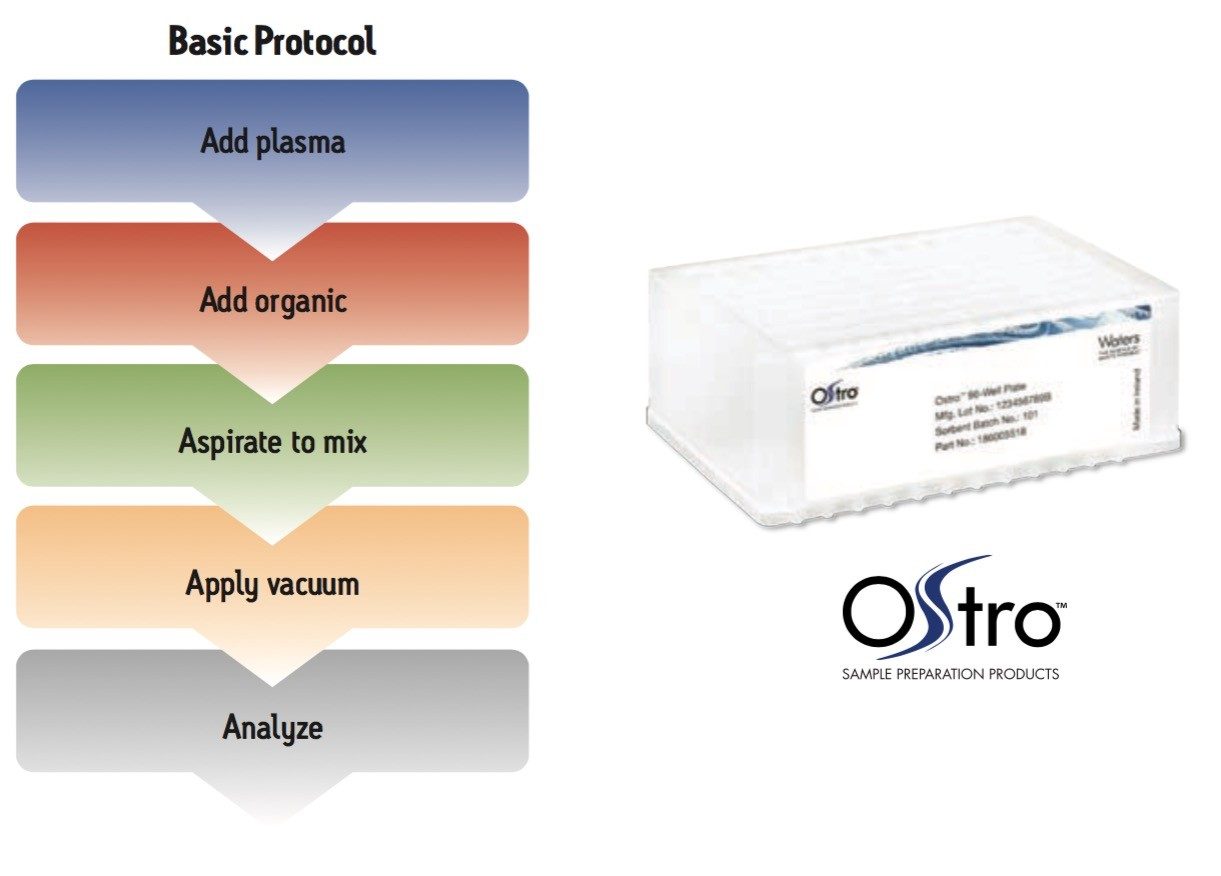
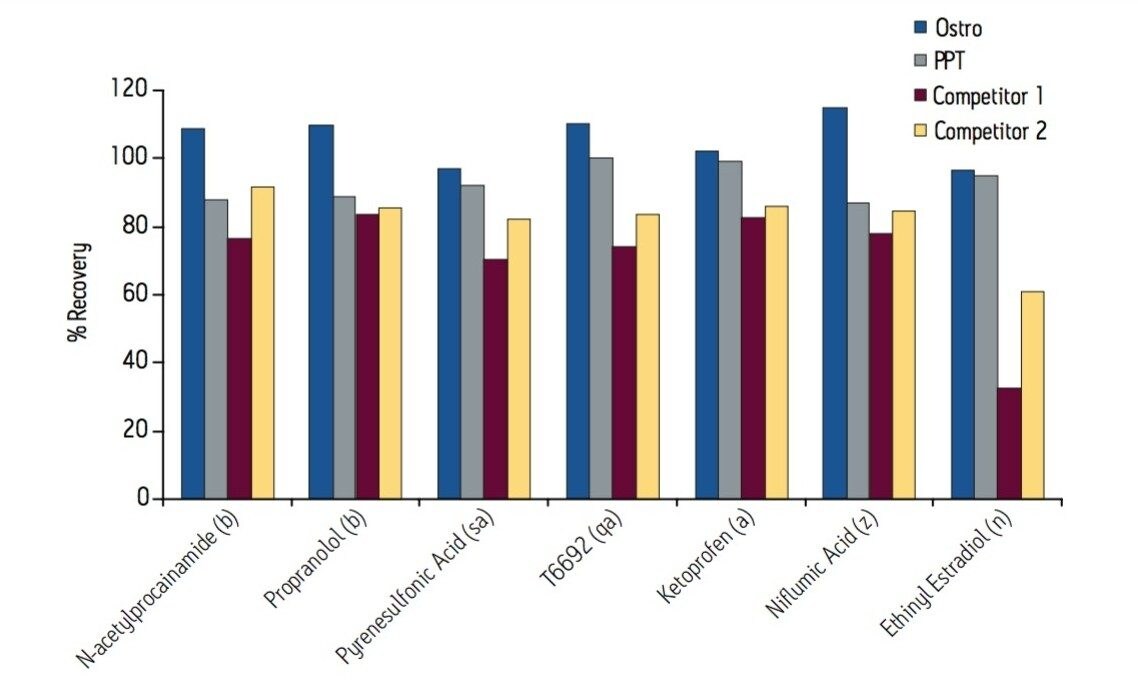
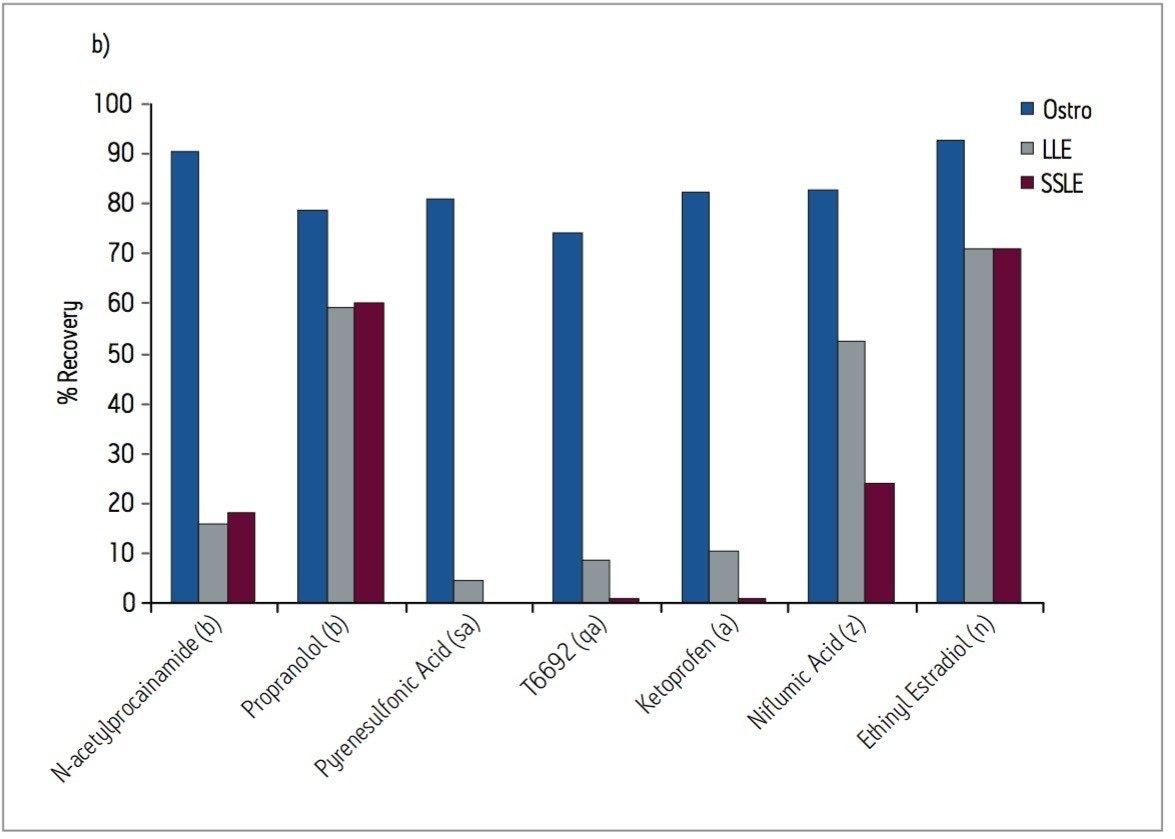
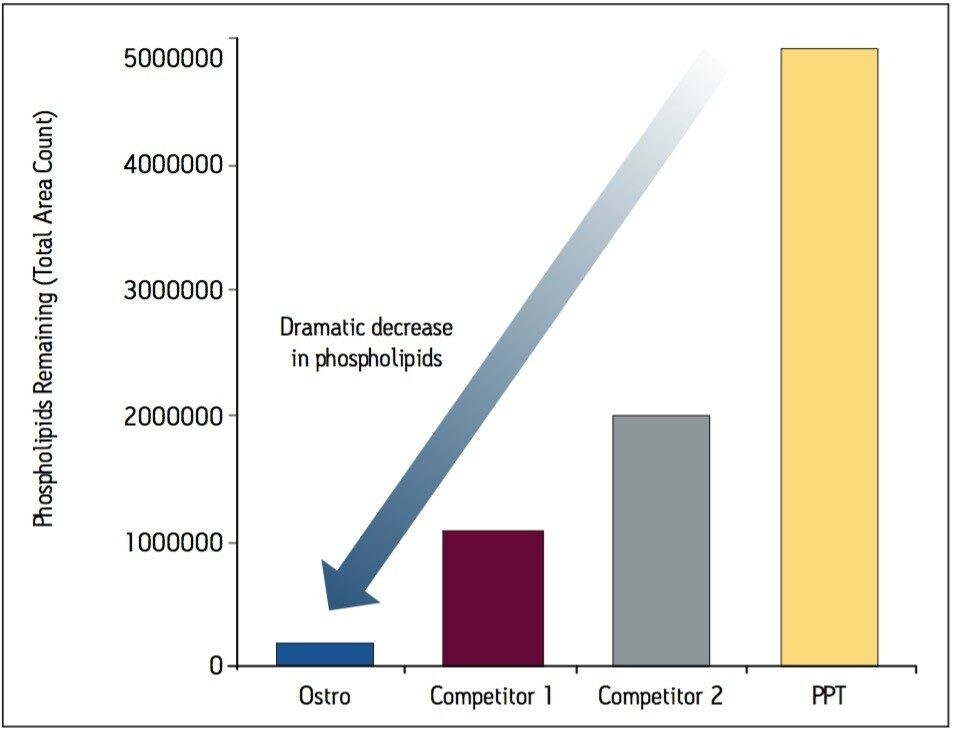
Samples were processed on the Ostro 96-well plate using the generic, simple protocol (Figure 1) provided by the manufacturer and a set of diverse analytes. Ostro sample preparation products provided overall significantly better recovery for the analytes tested (Figures 2a and b) than PPT, LLE, SSLE and competitive PL removal plates. The Ostro 96-well plate also removed significantly more phospholipids than the current phospholipid removal products on the market, PPT, LLE, and SSLE (Figures 3a and 3b.) In addition, not only was the level of PL removal better, but PLs were removed in a significantly more reproducible manner when Ostro Sample Preparation Products were used versus competitive PL removal devices (Figure 4). The average RSD for lipid removal across six sources of human plasma using Ostro was <1%, while competitive plates exhibited RSD’s between 24% and 41% for lipid removal.
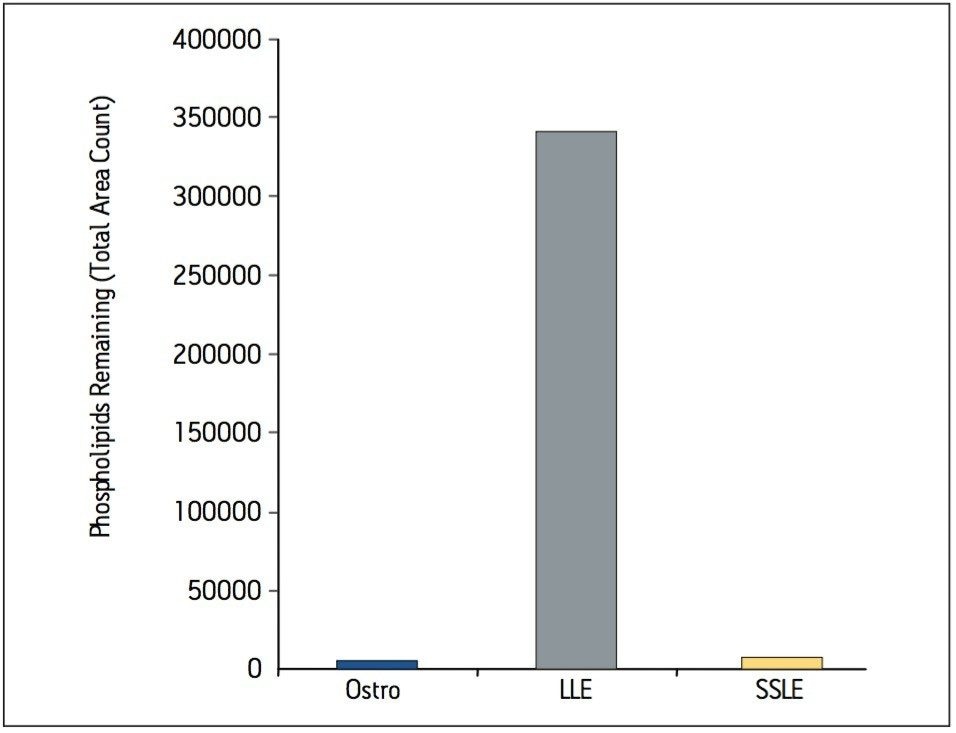
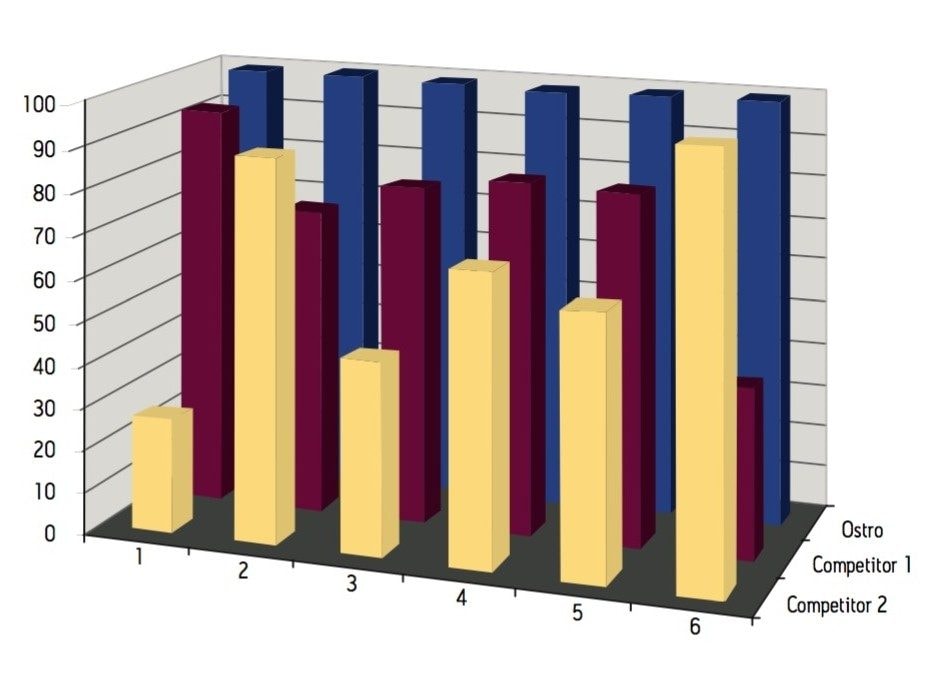
Providing cleaner, more reproducible extracts than competitive phospholipid removal devices or techniques, Ostro allows for more robust analyses and increased sample throughput. Requiring minimal to no method development, this technology can be rapidly implemented in order to improve laboratory workflow. Method simplicity, reproducibility, and highly efficient phospholipid removal make this technique an attractive alternative to liquid-liquid extraction or protein precipitation for obtaining higher quality data from large numbers of samples. In addition, the generic nature of this device and the significant clean-up provided facilitate productivity increases by reducing system downtime.
720003730, September 2010