Increasing Consistency Across Biopharmaceutical Labs with the ACQUITY Premier LC Platform
This is an Application Brief and does not contain a detailed Experimental section.
Abstract
Assessment of new technology is frequently encountered in the lifecycle management of drug products. As part of these evaluations, studies are typically performed to ensure performance and results are comparable with legacy methods to ensure data continuity. The ACQUITY Premier LC System featuring MaxPeak High Performance Surfaces was recently introduced as a flexible LC platform to support development and manufacturing activities. In this study, we evaluate the ACQUITY Premier System and its ability to reproduce results across different LC platforms using a legacy HILIC-based method. Results of a released glycan analysis showed comparable results when migrated between two ACQUITY Premier LC systems as well as with legacy data. Peak area % was observed to be comparable with less than 0.5% difference in relative peak area for glycan species between LC platforms and calculated glucose units (GU) values were determined to be within 0.1 unit of each other when compared to legacy values found in the Scientific Library within waters_connect. The comparable results across LC platforms and with the legacy method demonstrate that the ACQUITY Premier LC System is well suited to supporting the analytical needs in the development and manufacturing of biopharmaceuticals and offers compatibility with legacy methods.
Benefits
The ACQUITY Premier with MaxPeak High Performance Surfaces (HPS) Technology offers:
- The ability to acquire comparable results across different platforms
- Compatibility with legacy methods and scientific libraries
- Flexibility to support upstream and downstream lab activities
Introduction
Migration of methods across different LC platforms is commonly encountered in the biopharmaceutical industry as drug candidates progress from early stage to late stage development and manufacturing. This is in part by design as the instrument needs of supporting labs will differ across an organization. As drug candidates move through the pipeline, critical quality attributes (CQAs) are identified and monitored to ensure consistency in the manufacturing process, and that drug products are safe and efficacious. In this aspect, the need for methods to deliver consistent and accurate results across labs is critical as acceptance criteria cannot be readily changed once validated.
Recently, Waters introduced the ACQUITY Premier LC System with MaxPeak High Performance Surfaces (HPS) Technology as a flexible LC platform that can be readily deployed across labs to support development and manufacturing activities with improved chromatographic performance towards metal-sensitive analytes. This is accomplished through the introduction of the innovative MaxPeak HPS Technology which is engineered to minimize analyte/surface interaction of metal-sensitive analytes within the LC fluidic path.1–3 The purpose of this study is to demonstrate that the ACQUITY Premier LC Technology is robust and capable of delivering consistent results across LC platforms and is well suited to support the analytical needs of labs across an organization. As part of this evaluation, a HILIC-based method will be compared between two ACQUITY Premier LC platforms representative of a method being transferred across supporting labs.
Results and Discussion
Glycosylation of biotherapeutics can impact drug safety, efficacy, and stability, therefore the glycoprofile is typically monitored with specific species designated as CQAs as methods migrate through the product pipeline.4 Glycosylation is commonly assessed through the analysis of released and fluorescently labeled N-linked glycans via hydrophilic interaction chromatography (HILIC) coupled to fluorescence (FLR) and/or MS detection to provide comprehensive information including identity and relative abundance of glycan species.5,6
These methods, in part, rely on scientific libraries or online databases for glycan identification. In this aspect, results need to be consistent across LC platforms to facilitate comparison and ensure data continuity with legacy methods. Given this, a HILIC-based method based on the established Waters GU library will be used to evaluate the ACQUITY Premier LC Systems ability to deliver consistent results across LC platforms.
Briefly, the RapiFluor-MS Glycan Performance Test standard (p/n 186007983) was separated using the ACQUITY Premier Glycan BEH Amide Column, 1.7 µm, 130 Å, 2.1 x 150 mm (p/n 186009524) using a 0.6% B/min 35-minute gradient on two different ACQUITY Premier LC Systems. The BioAccord System with an ACQUITY Premier LC was used as an LC platform in the development of a method in the analysis of released glycans. As part of the waters_connect informatics platform, which controls the BioAccord System, the integrated glycan workflow and database can be readily applied in the identification of individual glycan species (see Application note 720007261EN for experimental details). The same method was performed on an ACQUITY Premier LC System controlled by Empower 3 representative of a system in a supporting lab the method is to be transferred to. For this system, complementary mass data was acquired using an ACQUITY QDa Mass Detector configured in-line post fluorescent detector as an orthogonal detection technique. Glycosylation profiles, relative abundance, RT, and GU values were used as metrics to evaluate comparability between systems.
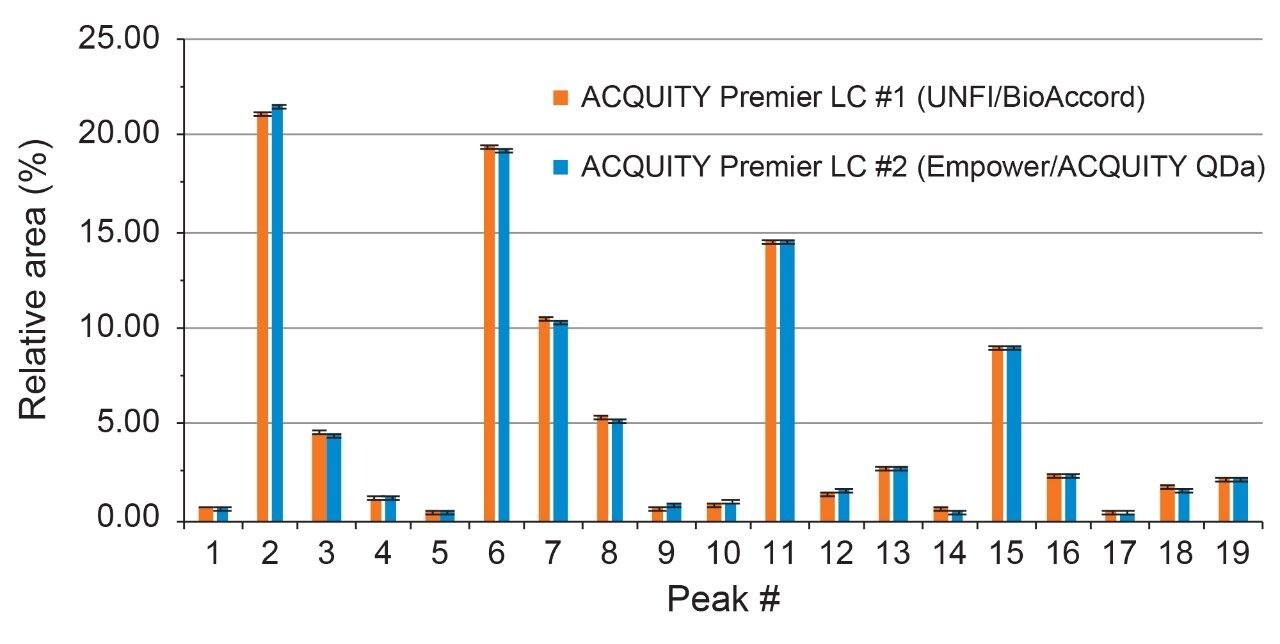
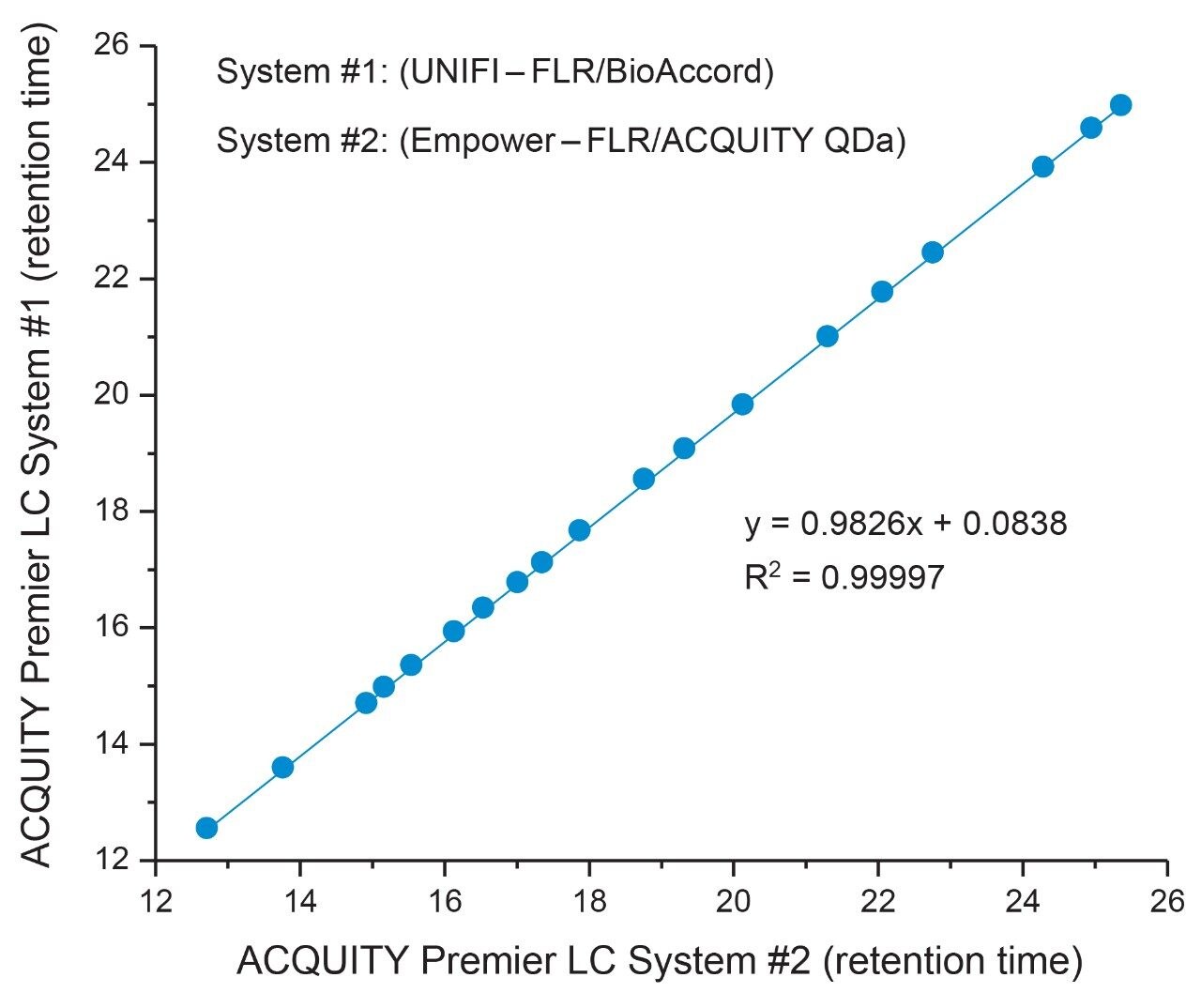
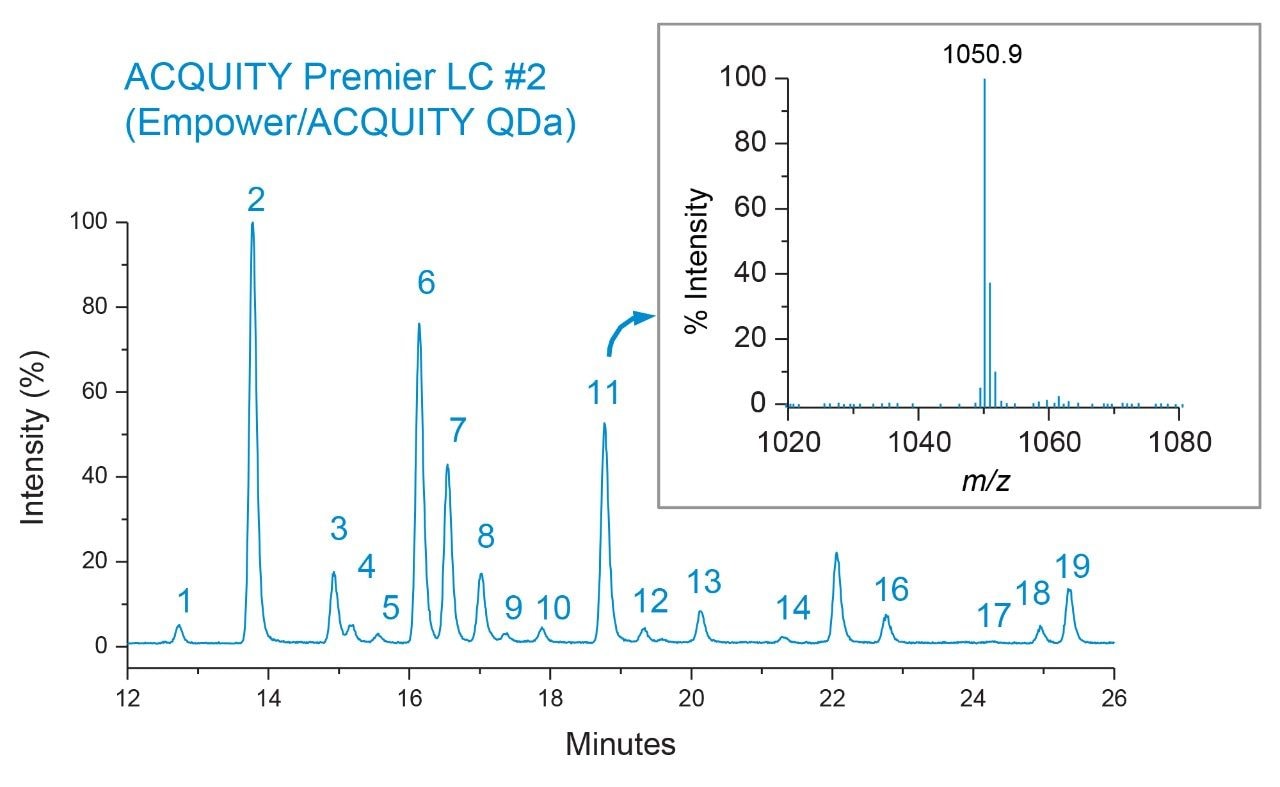
As shown in Figure 1, qualitative assessment of glycosylation profiles showed a high degree of comparability with respect to peak intensity, retention time, and species detected when compared across both platforms. Upon closer inspection, peak area was observed to be well preserved between LC platforms with peak % area differences calculated to be <0.5% for the same glycan species across platforms (Figure 2). The highly comparable peak area observed across both platforms indicates the ACQUITY Premier LC System is able to deliver consistent results for a given assay performed on different LC platforms, a quality highly desired in regulated labs where methods need to be robust and accurate and are often times deployed in different labs. Further inspection of the data showed a negligible systematic retention shift of 0.08 minutes when the method was performed on the Empower/ACQUITY QDa System as indicated by the y-intercept offset when comparing retention times between platforms (Figure 3). This observation may be attributed to minor variability in system dwell volume and/or method differences such as mobile phase composition. While minor, these differences can be corrected through the use of a reference standard to normalize response and facilitate direct comparison across platforms and/or historical data.
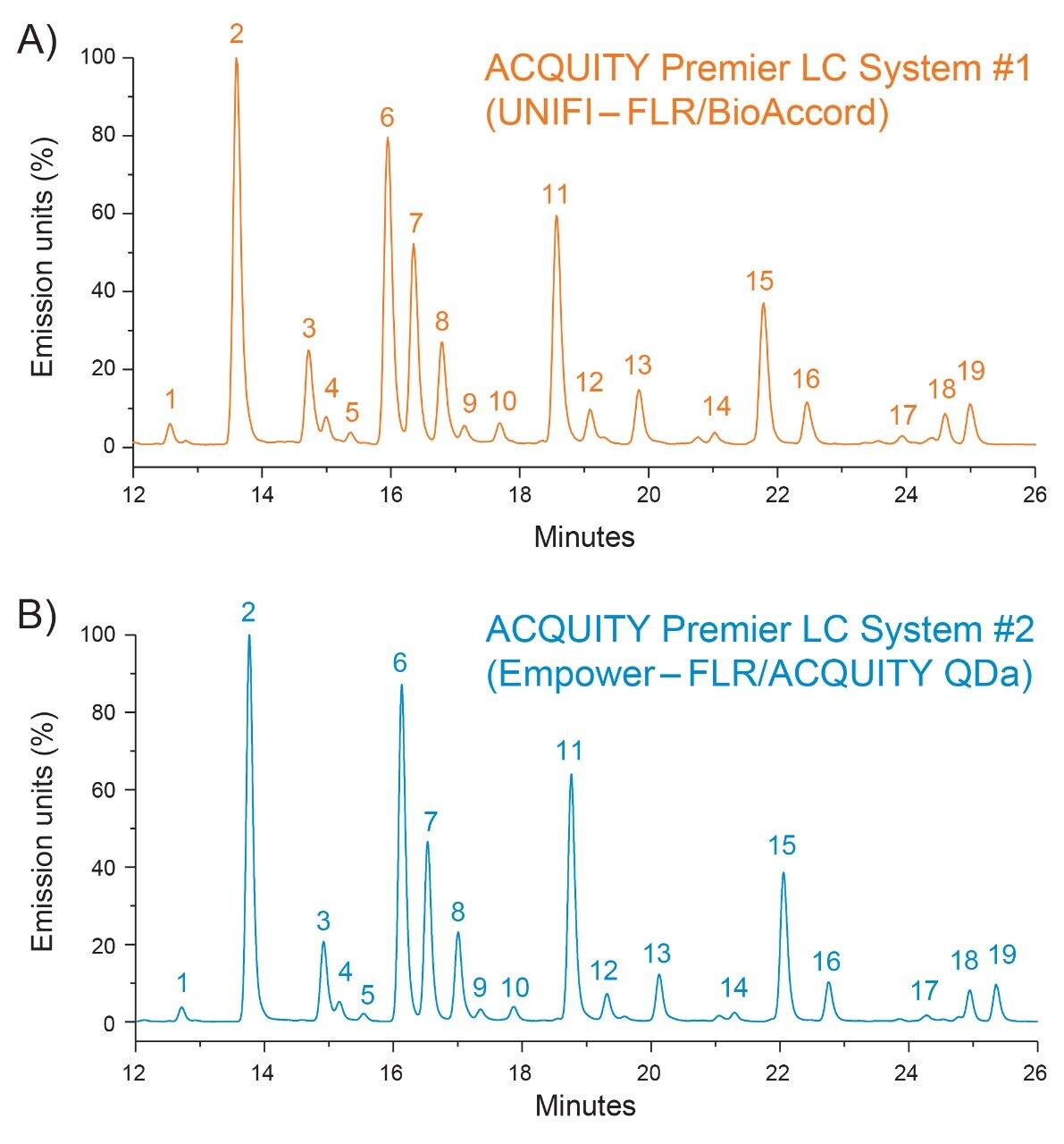
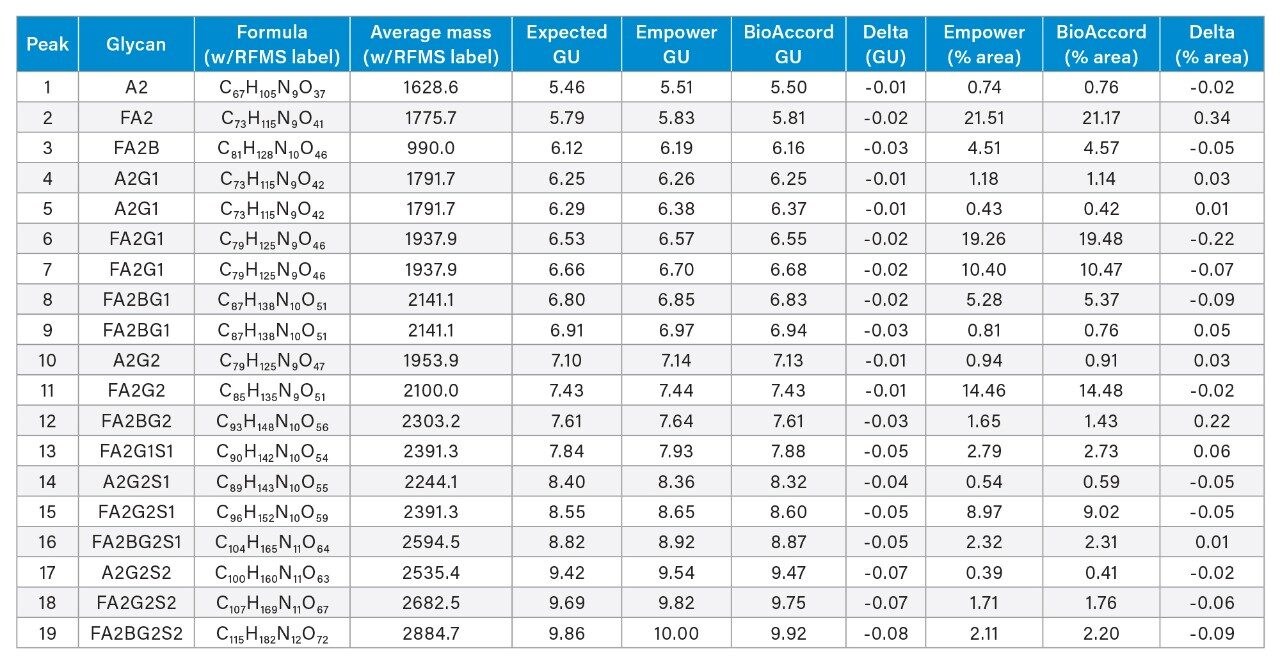
To demonstrate this principle, a dextran calibration ladder was used to generate a glucose unit (GU) value based on retention time (data not shown) using the Waters GU Library Method. As shown in Table 1, the GU values generated between both platforms showed a high degree of comparability with less than a 0.1 difference observed between systems and the Waters GU Library expected value. The GU value which is an integral part of the Waters GU Library is designed to facilitate glycan peak identification in conjunction with mass information.7 An example of this is shown for peak 11 (GU=7.44) which was observed to have a charge state of 1051 m/z observed in the MS spectrum acquired by the ACQUITY QDa Mass Detector (Figure 4 inset). Referring to Table 1, this is in good agreement with the Waters GU Library mass information and corresponds to the [M]+2H charge state of the FA2G2 glycan species (average mass = 2100 Da) further illustrating that the ACQUITY Premier LC Technology can deliver comparable results to legacy methods ensuring data continuity over a products lifecycle. Collectively, these results demonstrate that the ACQUITY Premier LC Technology is robust and capable of delivering consistent results across LC platforms in support of development and manufacturing lab activities.
Conclusion
LC instrument portfolios within the biopharmaceutical industry are generally observed to span a breadth of configurations that vary in performance and specifications. As part of lifecycle management, it is necessary to evaluate an instruments ability to deliver consistent and comparable results across LC platforms. This study demonstrates the ACQUITY Premier LC System is capable of reproducing results between two different ACQUITY Premier LC Systems for a HILIC-based assay while offering comparable performance to legacy methods. The high degree of consistency observed in peak area %, retention time, and calculated GU value demonstrate the ACQUITY Premier System is well-suited to support the development and manufacturing activity of biopharmaceuticals.
References
- Delano, et al. Using Hybrid Organic-Inorganic Surface Technology to Mitigate Analyte Interactions with Metal Surfaces in UHPLC. Anal. Chem. 2021, 93, 14, 5773–5781.
- Birdsall et al. Reducing Metal-Ion Mediated Adsorption of Acidic Peptides in RPLC-Based Assays using Hybrid Silica Chromatographic Surfaces. Journal of Chromatography B. 2021; 1179.
- Liu X., Lauber M. MaxPeak High Performance Surfaces Technology Improves HILIC Profiling of Released N-Glycans. Waters Application Note, 2021. 720007263EN.
- Higel F, et al. N-glycans of Complex Glycosylated Biopharmaceuticals and Their Impact on Protein Clearance. European Journal of Pharmaceutics and Biopharmaceutics. 2019; 139, 123–131.
- Yu, Y. Applying a Novel Glycan Tagging Reagent, RapiFluor-MS, and an Integrated UPLC-FLR/QTof MS System for Low Abundant N-Glycan Analysis. Waters Application Note, 2015. 720005383EN.
- Zhang X, Reed C, Shion H, Alley W, Birdsall R, Yu Y. Increasing Productivity and Confidence for N-linked Glycan Analysis of Biosimilars Using the BioAccord System. Waters Application Note, 2019. 720006545EN.
- Zhang X, Kellett J, Birdsall R, Yu Y. Improving Released N-Glycan Analysis in Biotherapeutic Development Using the ACQUITY Premier Solution with MaxPeak High Performance Surfaces (HPS) Technology. Waters Application Note, 2021. 720007261EN.
720007415, November 2021