This is an Application Brief and does not contain a detailed Experimental section.
Increasing demands on analytical labs across industry requires LC platforms to be utilized in an efficient manner to increase productivity. In this study, we evaluate the compatibility of the Waters ACQUITY Premier System with a broad set of LC techniques to determine its applicability as an LC platform that can be broadly deployed to support biopharmaceutical labs. Specifically, ion-exchange, size-exclusion, and hydrophobic interaction chromatography techniques are evaluated as complementary techniques to those already established to exhibit performance gains with MaxPeak High Performance Surfaces (HPS) Technology. This study demonstrates that the ACQUITY Premier System is compatible with methods that incorporate high-ionic strength mobile phases and can reliably run established methods with a high degree of comparability facilitating easier method transfer between LC platforms. In summary, the Waters ACQUITY Premier System with MaxPeak HPS Technology offers a flexible LC platform that can be broadly deployed across labs to support the analytical needs in the development and manufacturing of biotherapeutics.
Characterization and monitoring of biomolecules frequently requires employing orthogonal methods that span multiple techniques in the development and manufacturing of biopharmaceutical products. With increasing demands on productivity to serve pipelines, labs require LC platforms that can be utilized efficiently and offer broad compatibility with techniques used across an organization. Recently, Waters introduced the ACQUITY Premier System featuring MaxPeak High Performance Surfaces (HPS) Technology. The MaxPeak HPS Technology, which is engineered to reduce adsorptive losses due to analyte/surface interaction, has proven effective in the analysis of biomolecules with improved chromatographic performance of metal sensitive analytes using techniques including RPLC, HILIC, and IP-RPLC.1-3 With demonstrable performance gains established in these techniques, the question arises how the ACQUITY Premier System will perform in a broader context of LC-based techniques. More specifically, biotherapeutics frequently use methods that incorporate high-ionic strength mobile phases such as size-exclusion chromatography (SEC), ion-exchange chromatography (IEX), and hydrophobic interaction chromatography (HIC) as part of development and manufacturing activity. The goal of this study is to evaluate the ACQUITY Premier System as a flexible LC platform that can be broadly deployed across labs to support the analytical needs in the characterization and manufacturing of biotherapeutics.
The purpose of this study is to determine if the ACQUITY Premier System is compatible with methods that incorporate high-ionic strength mobile phases and if MaxPeak HPS technology offers additional benefit in terms of chromatographic performance under these conditions. To this end, IEX-, SEC-, and HIC-based methods were chosen as representative methods frequently used in the biopharmaceutical industry not yet evaluated with the ACQUITY Premier System. For this study, an ACQUITY UPLC H-Class PLUS Bio System was used to establish baseline performance to facilitate comparative discussion when using the same method on an equivalent ACQUITY Premier System.
IEX chromatography is a common technique used in industry to gain insight into structure and charge profile of biotherapeutics. This technique can be deployed using either salt or pH gradients to separate anionic and/or cationic charge variants and is available in “weak” and “strong” formats. The versatility of IEX and its general use as an impurity profiling technique makes it an ideal candidate to evaluate the flexibility of the ACQUITY Premier System as an LC platform that can be broadly deployed across an organization to support development and manufacturing activities. For this study, cetuximab, a commercially available mAb-based therapeutic, was analyzed under IEX conditions using a salt gradient prepared in 20 mM HEPES. Separations were performed on the Waters BioResolve SCX mAb Column (PN 186009058) using an optimized gradient from 25–65 mM NaCl prepared in 20 mM HEPES, pH 6.7. As shown in Figure 1A, from a qualitative standpoint, the ACQUITY Premier System was able to resolve the charge profile with the same fidelity as the conventional LC System. Further inspection of the chromatograms from a quantitative standpoint, as shown Figure 1B, indicates the separations are statistically indistinguishable from each other with respect to retention time (RT), resolving power (in terms of p/v), and peak capacity. The comparable performance exhibited by the ACQUITY Premier System was also observed in separations performed with MES buffer as well as pH gradients when using Waters IonHance CX-MS pH Buffer (PN 176004498) (data not shown). These initial results indicate the ACQUITY Premier System is a platform that provides comparable performance to conventional instruments and offers broader applicability with regards to LC techniques deployed in biopharmaceutical labs.

SEC as a technique is unique in that it is performed under isocratic conditions to effectively separate analytes based on their size via their accessibility to the porous network of the stationary phase. This technique has proven to be beneficial for biopharmaceuticals as it can be performed with “compatible” or non-denaturing mobile phases to gain insight into product/process related impurities such as aggregation or degradants. As an isocratic method, analytes analyzed using SEC are under continuous flow from injection to detection and do not adsorb to the stationary phase. In this regard SEC has increased sensitivity towards adsorption phenomena which can impact peak shape and overall performance making it an ideal test case to determine if MaxPeak HPS Technology offers an advantage over traditional LC platforms. To determine this, the Waters mAb Size Variant Standard (PN 186009429), comprised of humanized NIST mAb RM8671 and non-reduced IdeS digested NIST mAb fragments, was analyzed using an ACQUITY Protein BEH SEC Column (PN 186008471). Analysis was carried out using isocratic conditions typical of industry (50 mM Na2HPO4/200 mM KCl, pH 6.9) at a flow rate of 0.300 ml/min. As shown in Figure 2A, the ACQUITY Premier System was observed to have comparable performance in terms of overall profile when compared to the conventional LC platform while exhibiting better performance in terms of resolving the “clip” species from the native peak (inset) with a 34% improvement in peak-to-valley (p/v) ratio (1.41 vs 1.90). It should be noted that the observed performance gain may be specific to the standard as the resolution for higher (HMWS) and lower (LMWS) molecular weight species were determined to be statistically the same. Overall, however, the data suggests MaxPeak HPS Technology is largely compatible with existing methods that utilize conventional LC hardware while offering the possibility of additional benefit in some instances for SEC techniques deployed in the development and manufacturing of biopharmaceuticals.

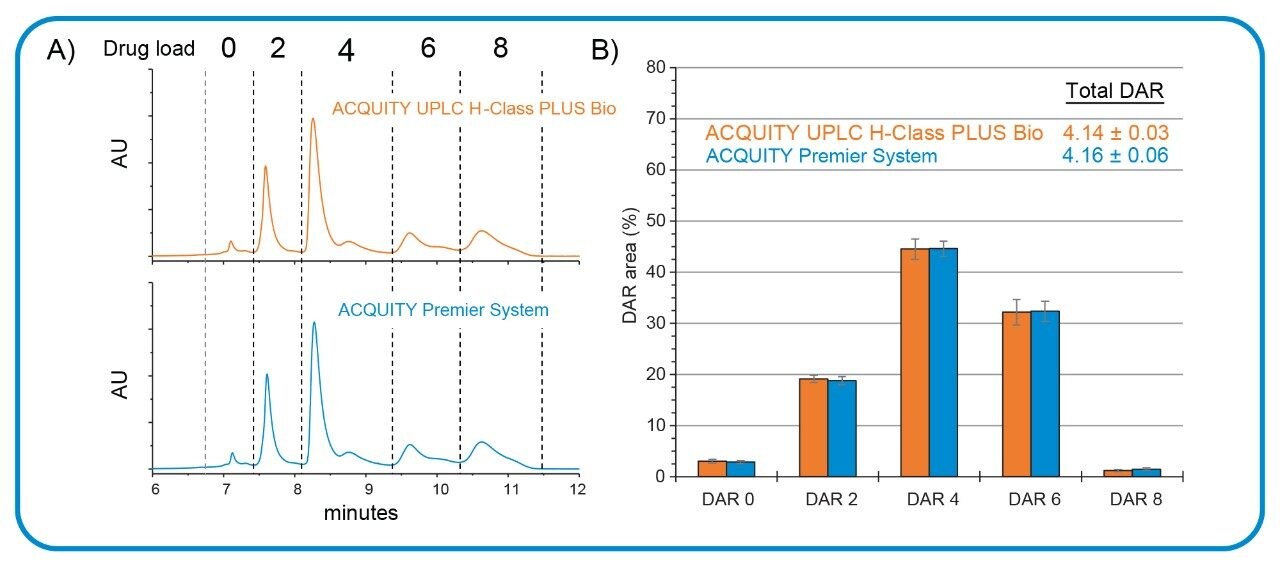
HIC is a technique frequently deployed in the determination of critical attributes of antibody drug-conjugates (ADCs). As a non-denaturing method, HIC can preserve the drug distribution profile of cysteine-conjugated ADCs allowing for accurate determination of the drug-antibody-ratio (DAR) value used to assess therapeutic safety and efficacy. This technique, which relies on high-ionic strength gradients, serves as an ideal test case to evaluate the ACQUITY Premier System performance under more demanding conditions. In this study, an ADC surrogate was analyzed with the Waters Protein-Pak Hi Res HIC Column (PN 186007583) using a 10 minute, high-ionic strength (2 M) gradient prepared from ammonium sulfate. As shown in Figure 3A, the chromatographic profile achieved using the ACQUITY Premier System agrees well with the profile from the conventional LC system. Closer inspection of the individual peak area, as indicated by the dashed lines, demonstrates that the separations are statistically indistinguishable from each other based on the standard deviation of three-replicate runs (Figure 3B). These results demonstrate the ACQUITY Premier System is able to perform reproducibly under demanding conditions and offer comparable performance to conventional LC systems enabling easier method transfer between platforms.
The compatibility of Waters ACQUITY Premier System with a broader set of techniques commonly encountered in the biopharmaceutical industry is clearly demonstrated by the highly comparable performance achieved for applications (IEX, SEC, HIC) evaluated in this study when compared to existing platforms. Quantitively, results were largely indistinguishable between LC platforms suggesting transfer of legacy methods is feasible. In this study, MaxPeak HPS Technology did not offer a particular advantage for techniques that incorporate high-ionic strength mobile phases, a result not un-expected as the ionic strength of the mobile phases are likely to mitigate analyte/surface adsorption phenomena. More importantly, this study demonstrates Waters ACQUITY Premier System with MaxPeak HPS Technology offers a flexible LC platform that can be broadly deployed across labs to support the analytical needs in the characterization, development, and manufacturing of biotherapeutics.
720007286, Revised December 2021